Biochemical and Functional Characterization of Glycoside Hydrolase Family 16 Genes in Aedes aegypti Larvae: Identification of the Major Digestive β-1,3-Glucanase
- PMID: 30873040
- PMCID: PMC6403176
- DOI: 10.3389/fphys.2019.00122
Biochemical and Functional Characterization of Glycoside Hydrolase Family 16 Genes in Aedes aegypti Larvae: Identification of the Major Digestive β-1,3-Glucanase
Abstract
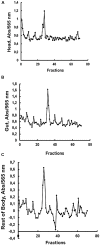
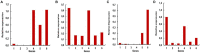
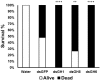
Insect β-1,3-glucanases belong to Glycoside Hydrolase Family 16 (GHF16) and are involved in digestion of detritus and plant hemicellulose. In this work, we investigated the role of GHF16 genes in Aedes aegypti larvae, due to their detritivore diet. Aedes aegypti genome has six genes belonging to GHF16 (Aae GH16.1 - Aae GH16.6), containing two to six exons. Sequence analysis suggests that five of these GHF16 sequences (Aae GH16.1, 2, 3, 5, and 6) contain the conserved catalytic residues of this family and correspond to glucanases. All genomes of Nematocera analyzed showed putative gene duplications corresponding to these sequences. Aae GH16.4 has no conserved catalytic residues and is probably a β-1,3-glucan binding protein involved in the activation of innate immune responses. Additionally, Ae. aegypti larvae contain significant β-1,3-glucanase activities in the head, gut and rest of body. These activities have optimum pH about 5-6 and molecular masses between 41 and 150 kDa. All GHF16 genes above showed different levels of expression in the larval head, gut or rest of the body. Knock-down of AeGH16.5 resulted in survival and pupation rates lower than controls (dsGFP and water treated). However, under stress conditions, severe mortalities were observed in AeGH16.1 and AeGH16.6 knocked-down larvae. Enzymatic assays of β-1,3-glucanase in AeGH16.5 silenced larvae exhibited lower activity in the gut and no change in the rest of the body. Chromatographic activity profiles from gut samples after GH16.5 silencing showed suppression of enzymatic activity, suggesting that this gene codes for the digestive larval β-1,3-glucanase of Ae. aegypti. This gene and enzyme are attractive targets for new control strategies, based on the impairment of normal gut physiology.
Keywords: Aedes aegypti; GHF16; Glycoside Hydrolase Family 16; digestion; immunity; knock-down; β-1,3-glucanase.
Figures




References
-
- Avissar Y. J., Margalit J., Spielman A. (1994). Incorporation of body components of diverse microorganisms by larval mosquitoes. J. Am. Mosq. Control Assoc. 10 45–50. - PubMed
LinkOut - more resources
Full Text Sources

