Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group
- PMID: 30873135
- PMCID: PMC6401651
- DOI: 10.3389/fmicb.2019.00302
Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group
Abstract
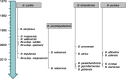
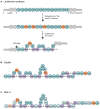
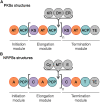
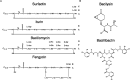
Over the last seven decades, applications using members of the Bacillus subtilis group have emerged in both food processes and crop protection industries. Their ability to form survival endospores and the plethora of antimicrobial compounds they produce has generated an increased industrial interest as food preservatives, therapeutic agents and biopesticides. In the growing context of food biopreservation and biological crop protection, this review suggests a comprehensive way to visualize the antimicrobial spectrum described within the B. subtilis group, including volatile compounds. This classification distinguishes the bioactive metabolites based on their biosynthetic pathways and chemical nature: i.e., ribosomal peptides (RPs), volatile compounds, polyketides (PKs), non-ribosomal peptides (NRPs), and hybrids between PKs and NRPs. For each clade, the chemical structure, biosynthesis and antimicrobial activity are described and exemplified. This review aims at constituting a convenient and updated classification of antimicrobial metabolites from the B. subtilis group, whose complex phylogeny is prone to further development.
Keywords: Bacillus subtilis group; bacteriocins; biocontrol; biosynthetic pathways; lipopeptides; polyketides; siderophores; volatile.
Figures




References
-
- Agrios G. N. (1988). “1 - INTRODUCTION TO PLANT PATHOLOGY,” in Plant Pathology, 3rdEdition Edn, ed. Agrios G. N. (New York, NY: Academic Press; ), 3–39. 10.1016/B978-0-12-044563-9.50005-0 - DOI
-
- Alamri S. A. (2015). Enhancing the efficiency of the bioagent Bacillus subtilis JF419701 against soil-borne phytopathogens by increasing the productivity of fungal cell wall degrading enzymes. Arch. Phytopathol. Plant Prot. 48 159–170. 10.1080/03235408.2014.884671 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

