Rapid Downregulation of DAB2 by Toll-Like Receptor Activation Contributes to a Pro-Inflammatory Switch in Activated Dendritic Cells
- PMID: 30873168
- PMCID: PMC6400992
- DOI: 10.3389/fimmu.2019.00304
Rapid Downregulation of DAB2 by Toll-Like Receptor Activation Contributes to a Pro-Inflammatory Switch in Activated Dendritic Cells
Abstract
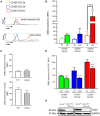
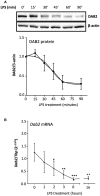
Dendritic cells (DCs) are pivotal in regulating tolerogenic as well as immunogenic responses against microorganisms by directing both the innate and adaptive immune response. In health, phenotypically different DC subsets found in the gut mucosa are maintained in their tolerogenic state but switch to a pro-inflammatory phenotype during infection or chronic autoinflammatory conditions such as inflammatory bowel disease (IBD). The mechanisms that promote the switch among the mucosal DCs from a tolerogenic to an immunogenic, pro-inflammatory phenotype are incompletely understood. We hypothesized that disabled homolog 2 (DAB2), recently described as a negative regulator of DC immunogenicity during their development, is regulated during intestinal inflammation and modulates mucosal DC function. We show that DAB2 is highly expressed in colonic CD11b+CD103- DCs, a subset known for its capacity to induce inflammatory Th1/Th17 responses in the colon, and is downregulated predominantly in this DC subset during adoptive T cell transfer colitis. Administration of Dab2-deficient DCs (DC2.4 Dab2-/- cells) modulated the course of DSS colitis in wild-type mice, enhanced mucosal expression of Tnfa, Il6, and Il17a, and promoted neutrophil recruitment. In bone-marrow derived dendritic cells (BMDC), DAB2 expression correlated with CD11b levels and DAB2 was rapidly and profoundly inhibited by TLR ligands in a TRIF- and MyD88-dependent manner. The negative modulation of DAB2 was biphasic, initiated with a quick drop in DAB2 protein, followed by a sustained reduction in Dab2 mRNA. DAB2 downregulation promoted a more functional and activated DC phenotype, reduced phagocytosis, and increased CD40 expression after TLR activation. Furthermore, Dab2 knockout in DCs inhibited autophagy and promoted apoptotic cell death. Collectively, our results highlight the immunoregulatory role for DAB2 in the intestinal dendritic cells and suggest that DAB2 downregulation after microbial exposure promotes their switch to an inflammatory phenotype.
Keywords: Dab2; colon; dendritic cells; immunoregulation; inflammation; small intestine.
Figures








Similar articles
-
A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity.J Immunol. 2011 Jun 1;186(11):6287-95. doi: 10.4049/jimmunol.1004036. Epub 2011 Apr 27. J Immunol. 2011. PMID: 21525388
-
Cisplatin induces tolerogenic dendritic cells in response to TLR agonists via the abundant production of IL-10, thereby promoting Th2- and Tr1-biased T-cell immunity.Oncotarget. 2016 Jun 7;7(23):33765-82. doi: 10.18632/oncotarget.9260. Oncotarget. 2016. PMID: 27172902 Free PMC article.
-
Disabled-2 (DAB2) Overexpression Inhibits Monocyte-Derived Dendritic Cells' Function in Vogt-Koyanagi-Harada Disease.Invest Ophthalmol Vis Sci. 2018 Sep 4;59(11):4662-4669. doi: 10.1167/iovs.18-24630. Invest Ophthalmol Vis Sci. 2018. PMID: 30267088
-
Emerging Roles of Disabled Homolog 2 (DAB2) in Immune Regulation.Front Immunol. 2020 Oct 15;11:580302. doi: 10.3389/fimmu.2020.580302. eCollection 2020. Front Immunol. 2020. PMID: 33178208 Free PMC article. Review.
-
Immunomodulation by semi-mature dendritic cells: a novel role of Toll-like receptors and interleukin-6.Int J Med Microbiol. 2010 Jan;300(1):19-24. doi: 10.1016/j.ijmm.2009.08.010. Epub 2009 Sep 24. Int J Med Microbiol. 2010. PMID: 19781988 Review.
Cited by
-
Microbial Symphony: Exploring the Role of the Gut in Osteoarthritis-Related Pain. A Narrative Review.Pain Ther. 2024 Jun;13(3):409-433. doi: 10.1007/s40122-024-00602-9. Epub 2024 Apr 27. Pain Ther. 2024. PMID: 38678155 Free PMC article. Review.
-
Functions of Dendritic Cells and Its Association with Intestinal Diseases.Cells. 2021 Mar 6;10(3):583. doi: 10.3390/cells10030583. Cells. 2021. PMID: 33800865 Free PMC article. Review.
-
Toll-Like Receptor-Mediated Activation of CD39 Internalization in BMDCs Leads to Extracellular ATP Accumulation and Facilitates P2X7 Receptor Activation.Front Immunol. 2019 Oct 31;10:2524. doi: 10.3389/fimmu.2019.02524. eCollection 2019. Front Immunol. 2019. PMID: 31736956 Free PMC article.
-
Photocurable Hydrogel Substrate-Better Potential Substitute on Bone-Marrow-Derived Dendritic Cells Culturing.Materials (Basel). 2022 May 5;15(9):3322. doi: 10.3390/ma15093322. Materials (Basel). 2022. PMID: 35591655 Free PMC article.
-
Disabled-2 (DAB2): A Key Regulator of Anti- and Pro-Tumorigenic Pathways.Int J Mol Sci. 2022 Dec 31;24(1):696. doi: 10.3390/ijms24010696. Int J Mol Sci. 2022. PMID: 36614139 Free PMC article. Review.
References
-
- Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, et al. . Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. (2003) 171:4984–9. 10.4049/jimmunol.171.10.4984 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

