Targeting Lipooligosaccharide (LOS) for a Gonococcal Vaccine
- PMID: 30873172
- PMCID: PMC6400993
- DOI: 10.3389/fimmu.2019.00321
Targeting Lipooligosaccharide (LOS) for a Gonococcal Vaccine
Abstract
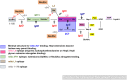
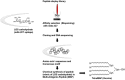
The increasing incidence of gonorrhea worldwide and the global spread of multidrug-resistant strains of Neisseria gonorrhoeae, constitute a public health emergency. With dwindling antibiotic treatment options, there is an urgent need to develop safe and effective vaccines. Gonococcal lipooligosaccharides (LOSs) are potential vaccine candidates because they are densely represented on the bacterial surface and are readily accessible as targets of adaptive immunity. Less well-understood is whether LOSs evoke protective immune responses. Although gonococcal LOS-derived oligosaccharides (OSs) are major immune targets, often they undergo phase variation, a feature that seemingly makes LOS less desirable as a vaccine candidate. However, the identification of a gonococcal LOS-derived OS epitope, called 2C7, that is: (i) a broadly expressed gonococcal antigenic target in human infection; (ii) a virulence determinant, that is maintained by the gonococcus and (iii) a critical requirement for gonococcal colonization in the experimental setting, circumvents its limitation as a potential vaccine candidate imposed by phase variation. Difficulties in purifying structurally intact OSs from LOSs led to "conversion" of the 2C7 epitope into a peptide mimic that elicited cross-reactive IgG anti-OS antibodies that also possess complement-dependent bactericidal activity against gonococci. Mice immunized with the 2C7 peptide mimic clear vaginal colonization more rapidly and reduce gonococcal burdens. 2C7 vaccine satisfies criteria that are desirable in a gonococcal vaccine candidate: broad representation of the antigenic target, service as a virulence determinant that is also critical for organism survival in vivo and elicitation of broadly cross-reactive IgG bactericidal antibodies when used as an immunogen.
Keywords: Lipooligosaccharide; N. gonorrhoeae; complement; peptide mimic; vaccine.
Figures

Similar articles
-
Preclinical Efficacy of a Lipooligosaccharide Peptide Mimic Candidate Gonococcal Vaccine.mBio. 2019 Nov 5;10(6):e02552-19. doi: 10.1128/mBio.02552-19. mBio. 2019. PMID: 31690678 Free PMC article.
-
Immunization against a saccharide epitope accelerates clearance of experimental gonococcal infection.PLoS Pathog. 2013;9(8):e1003559. doi: 10.1371/journal.ppat.1003559. Epub 2013 Aug 29. PLoS Pathog. 2013. PMID: 24009500 Free PMC article. Clinical Trial.
-
Synthetic DNA Delivery of an Optimized and Engineered Monoclonal Antibody Provides Rapid and Prolonged Protection against Experimental Gonococcal Infection.mBio. 2021 Mar 16;12(2):e00242-21. doi: 10.1128/mBio.00242-21. mBio. 2021. PMID: 33727348 Free PMC article.
-
Strategies for mimicking Neisserial saccharide epitopes as vaccines.Int Rev Immunol. 2001;20(2):229-50. doi: 10.3109/08830180109043036. Int Rev Immunol. 2001. PMID: 11878767 Review.
-
Biology of the Gonococcus: Disease and Pathogenesis.Methods Mol Biol. 2019;1997:1-27. doi: 10.1007/978-1-4939-9496-0_1. Methods Mol Biol. 2019. PMID: 31119614 Review.
Cited by
-
Hijacking Factor H for Complement Immune Evasion.Front Immunol. 2021 Feb 25;12:602277. doi: 10.3389/fimmu.2021.602277. eCollection 2021. Front Immunol. 2021. PMID: 33717083 Free PMC article. Review.
-
Addressing Sexually Transmitted Infections Due to Neisseria gonorrhoeae in the Present and Future.Microorganisms. 2024 Apr 28;12(5):884. doi: 10.3390/microorganisms12050884. Microorganisms. 2024. PMID: 38792714 Free PMC article. Review.
-
Recent Progress Towards a Gonococcal Vaccine.Front Cell Infect Microbiol. 2022 Apr 11;12:881392. doi: 10.3389/fcimb.2022.881392. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35480233 Free PMC article. Review.
-
Recent advances in understanding and combatting Neisseria gonorrhoeae: a genomic perspective.Fac Rev. 2021 Aug 27;10:65. doi: 10.12703/r/10-65. eCollection 2021. Fac Rev. 2021. PMID: 34557869 Free PMC article. Review.
-
The serogroup B meningococcal outer membrane vesicle-based vaccine 4CMenB induces cross-species protection against Neisseria gonorrhoeae.PLoS Pathog. 2020 Dec 8;16(12):e1008602. doi: 10.1371/journal.ppat.1008602. eCollection 2020 Dec. PLoS Pathog. 2020. PMID: 33290434 Free PMC article.
References
-
- Almonacid-Mendoza HL, Humbert MV, Dijokaite A, Cleary DW, Soo Y, Hung MC, et al. . Structure of the recombinant Neisseria gonorrhoeae adhesin complex protein (rNg-ACP) and generation of murine antibodies with bactericidal activity against gonococci. mSphere. (2018) 3:e00331–18. 10.1128/mSphere.00331-18 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

