Cytosolic Glucose-6-Phosphate Dehydrogenase Is Involved in Seed Germination and Root Growth Under Salinity in Arabidopsis
- PMID: 30873191
- PMCID: PMC6401624
- DOI: 10.3389/fpls.2019.00182
Cytosolic Glucose-6-Phosphate Dehydrogenase Is Involved in Seed Germination and Root Growth Under Salinity in Arabidopsis
Abstract
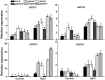
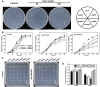
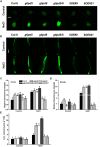
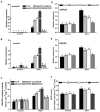
Glucose-6-phosphate dehydrogenase (G6PDH or G6PD) is the key regulatory enzyme in the oxidative pentose phosphate pathway (OPPP). The cytosolic isoforms including G6PD5 and G6PD6 account for the major part of the G6PD total activity in plant cells. Here, we characterized the Arabidopsis single null mutant g6pd5 and g6pd6 and double mutant g6pd5/6. Compared to wild type, the mutant seeds showed a reduced germination rate and root elongation under salt stress. The seeds and seedlings lacking G6PD5 and G6PD6 accumulate more reactive oxygen species (ROS) than the wild type under salt stress. Cytosolic G6PD (cy-G6PD) affected the expression of NADPH oxidases and the G6PD enzymatic activities in the mutant atrbohD/F, in which the NADPH oxidases genes are disrupted by T-DNA insertion and generation of ROS is inhibited, were lower than that in the wild type. The NADPH level in mutants was decreased under salt stress. In addition, we found that G6PD5 and G6PD6 affected the activities and transcript levels of various antioxidant enzymes in response to salt stress, especially the ascorbate peroxidase and glutathione reductase. Exogenous application of ascorbate acid and glutathione rescued the seed and root phenotype of g6pd5/6 under salt stress. Interestingly, the cytosolic G6PD negatively modulated the NaCl-blocked primary root growth under salt stress in the root meristem and elongation zone.
Keywords: NADPH oxidases; NaCl; germination; glucose-6-phosphate dehydrogenase; reactive oxygen species; root system architecture.
Figures



Similar articles
-
Involvement of G6PD5 in ABA response during seed germination and root growth in Arabidopsis.BMC Plant Biol. 2019 Jan 30;19(1):44. doi: 10.1186/s12870-019-1647-8. BMC Plant Biol. 2019. PMID: 30700259 Free PMC article.
-
Cytosolic glucose-6-phosphate dehydrogenases play a pivotal role in Arabidopsis seed development.Plant Physiol Biochem. 2022 Sep 1;186:207-219. doi: 10.1016/j.plaphy.2022.07.017. Epub 2022 Jul 16. Plant Physiol Biochem. 2022. PMID: 35870442
-
Alternative splicing of Arabidopsis G6PD5 recruits NADPH-producing OPPP reactions to the endoplasmic reticulum.Front Plant Sci. 2022 Sep 2;13:909624. doi: 10.3389/fpls.2022.909624. eCollection 2022. Front Plant Sci. 2022. PMID: 36119606 Free PMC article.
-
Neuroprotection by glucose-6-phosphate dehydrogenase and the pentose phosphate pathway.J Cell Biochem. 2019 Sep;120(9):14285-14295. doi: 10.1002/jcb.29004. Epub 2019 May 24. J Cell Biochem. 2019. PMID: 31127649 Review.
-
Nitrogen Assimilation, Abiotic Stress and Glucose 6-Phosphate Dehydrogenase: The Full Circle of Reductants.Plants (Basel). 2016 May 11;5(2):24. doi: 10.3390/plants5020024. Plants (Basel). 2016. PMID: 27187489 Free PMC article. Review.
Cited by
-
Evaluation of protein's interaction and the regulatory network of some drought-responsive genes in Canola under drought and re-watering conditions.Physiol Mol Biol Plants. 2023 Aug;29(8):1085-1102. doi: 10.1007/s12298-023-01345-1. Epub 2023 Sep 1. Physiol Mol Biol Plants. 2023. PMID: 37829706 Free PMC article.
-
Roles of Reactive Oxygen Species and Mitochondria in Seed Germination.Front Plant Sci. 2021 Dec 9;12:781734. doi: 10.3389/fpls.2021.781734. eCollection 2021. Front Plant Sci. 2021. PMID: 34956279 Free PMC article. Review.
-
Natural elicitors enhanced suberin polyphenolic accumulation in wounded potato tuber tissues.Front Plant Sci. 2024 May 28;15:1384602. doi: 10.3389/fpls.2024.1384602. eCollection 2024. Front Plant Sci. 2024. PMID: 38867884 Free PMC article.
-
Epichloë gansuensis Increases the Tolerance of Achnatherum inebrians to Low-P Stress by Modulating Amino Acids Metabolism and Phosphorus Utilization Efficiency.J Fungi (Basel). 2021 May 17;7(5):390. doi: 10.3390/jof7050390. J Fungi (Basel). 2021. PMID: 34067720 Free PMC article.
-
Identification of the Cytosolic Glucose-6-Phosphate Dehydrogenase Gene from Strawberry Involved in Cold Stress Response.Int J Mol Sci. 2020 Oct 3;21(19):7322. doi: 10.3390/ijms21197322. Int J Mol Sci. 2020. PMID: 33023038 Free PMC article.
References
-
- Cardi M., Castiglia D., Ferrara M., Guerriero G., Chiurazzi M., Esposito S. (2015). The effects of salt stress cause a diversion of basal metabolism in barley roots: possible different roles for glucose-6-phosphate dehydrogenase isoforms. Plant Physiol. Biochem. 86 44–54. 10.1016/j.plaphy.2014.11.001 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

