Aqueous olefin metathesis: recent developments and applications
- PMID: 30873229
- PMCID: PMC6404410
- DOI: 10.3762/bjoc.15.39
Aqueous olefin metathesis: recent developments and applications
Abstract
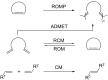
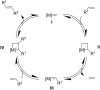
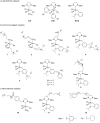
Olefin metathesis is one of the most powerful C-C double-bond-forming reactions. Metathesis reactions have had a tremendous impact in organic synthesis, enabling a variety of applications in polymer chemistry, drug discovery and chemical biology. Although challenging, the possibility to perform aqueous metatheses has become an attractive alternative, not only because water is a more sustainable medium, but also to exploit biocompatible conditions. This review focuses on the progress made in aqueous olefin metatheses and their applications in chemical biology.
Keywords: aqueous catalysis; artificial metalloenzymes; chemical biology; green chemistry; olefin metathesis; ruthenium catalysts; stapled peptides.
Figures
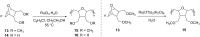











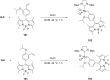

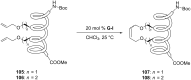
Similar articles
-
Sustainable concepts in olefin metathesis.Angew Chem Int Ed Engl. 2007;46(36):6786-801. doi: 10.1002/anie.200605099. Angew Chem Int Ed Engl. 2007. PMID: 17640026 Review.
-
Olefin metathesis for chemical biology.Curr Opin Chem Biol. 2008 Dec;12(6):767-73. doi: 10.1016/j.cbpa.2008.09.022. Epub 2008 Oct 18. Curr Opin Chem Biol. 2008. PMID: 18935975 Free PMC article. Review.
-
Tandem Catalysis Utilizing Olefin Metathesis Reactions.Chemistry. 2016 Jul 4;22(28):9440-54. doi: 10.1002/chem.201505136. Epub 2016 May 20. Chemistry. 2016. PMID: 27203528
-
The allylic chalcogen effect in olefin metathesis.Beilstein J Org Chem. 2010 Dec 23;6:1219-28. doi: 10.3762/bjoc.6.140. Beilstein J Org Chem. 2010. PMID: 21283554 Free PMC article.
-
Recent developments in olefin cross-metathesis.Angew Chem Int Ed Engl. 2003 Apr 29;42(17):1900-23. doi: 10.1002/anie.200200556. Angew Chem Int Ed Engl. 2003. PMID: 12730969
Cited by
-
Water-Accelerated Decomposition of Olefin Metathesis Catalysts.ACS Catal. 2023 Jan 3;13(2):1097-1102. doi: 10.1021/acscatal.2c05573. eCollection 2023 Jan 20. ACS Catal. 2023. PMID: 36714054 Free PMC article.
-
Selective Hydroboration-Oxidation of Terminal Alkenes under Flow Conditions.Chemistry. 2020 Sep 4;26(50):11423-11425. doi: 10.1002/chem.202001650. Epub 2020 Aug 6. Chemistry. 2020. PMID: 32329919 Free PMC article.
-
Olefin Metathesis in Water: Speciation of a Leading Water-Soluble Catalyst Pinpoints Challenges and Opportunities for Chemical Biology.J Am Chem Soc. 2025 Mar 19;147(11):9441-9448. doi: 10.1021/jacs.4c16700. Epub 2025 Mar 7. J Am Chem Soc. 2025. PMID: 40053839 Free PMC article.
-
Synthetic prodrug design enables biocatalytic activation in mice to elicit tumor growth suppression.Nat Commun. 2022 Jan 10;13(1):39. doi: 10.1038/s41467-021-27804-5. Nat Commun. 2022. PMID: 35013295 Free PMC article.
-
Olefin Metathesis in Continuous Flow Reactor Employing Polar Ruthenium Catalyst and Soluble Metal Scavenger for Instant Purification of Products of Pharmaceutical Interest.ACS Sustain Chem Eng. 2021 Dec 6;9(48):16450-16458. doi: 10.1021/acssuschemeng.1c06522. Epub 2021 Nov 22. ACS Sustain Chem Eng. 2021. PMID: 34900446 Free PMC article.
References
-
- Grubbs R H, Wenzel A G, O'Leary D J, et al., editors. Handbook of Metathesis. 2nd ed. 1–3. Weinheim, Germany: Wiley-VCH Verlag GmbH; 2015. - DOI
-
- Fürstner A, editor. Alkene Metathesis in Organic Synthesis. Vol. 1. Berlin, Heidelberg: Springer-Verlag; 1998. ((Topics in Organometallic Chemistry)). - DOI
Publication types
LinkOut - more resources
Full Text Sources
