Calcium and vitamin D supplementation and/or periodontal therapy in the treatment of periodontitis among Brazilian pregnant women: protocol of a feasibility randomised controlled trial (the IMPROVE trial)
- PMID: 30873290
- PMCID: PMC6402123
- DOI: 10.1186/s40814-019-0417-6
Calcium and vitamin D supplementation and/or periodontal therapy in the treatment of periodontitis among Brazilian pregnant women: protocol of a feasibility randomised controlled trial (the IMPROVE trial)
Erratum in
-
Correction to: Calcium and vitamin D supplementation and/or periodontal therapy in the treatment of periodontitis among Brazilian pregnant women: protocol of a feasibility randomised controlled trial (the IMPROVE trial).Pilot Feasibility Stud. 2020 Nov 27;6(1):187. doi: 10.1186/s40814-020-00727-6. Pilot Feasibility Stud. 2020. PMID: 33292810 Free PMC article.
Abstract
Background: Periodontitis is a common oral inflammation, which is a risk factor for adverse pregnancy outcomes. Intakes of vitamin D and calcium are inversely associated with occurrence and progression of periodontitis. This study aims to assess the feasibility of a multi-component intervention, including provision of milk powder supplemented with calcium and vitamin D and periodontal therapy (PT), for improving maternal periodontal health and metabolic and inflammatory profiles of low-income Brazilian pregnant women with periodontitis.
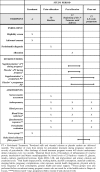
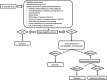
Methods: The IMPROVE trial is a feasibility randomised controlled trial (RCT) with a 2 × 2 factorial design with a parallel process evaluation. Pregnant women with periodontitis, aged 18-40 years and with < 20 gestational weeks (n = 120) were recruited and randomly allocated into four groups: (1) fortified sachet (vitamin D and calcium) and powdered milk plus PT during pregnancy, (2) placebo sachet and powdered milk plus PT during pregnancy, (3) fortified sachet (vitamin D and calcium) and powdered milk plus PT after delivery and (4) placebo sachet and powdered milk plus PT after delivery. Dentists and participants are blinded to fortification. Acceptability of study design, recruitment strategy, random allocation, data collection procedures, recruitment rate, adherence and attrition rate will be evaluated. Data on serum levels of vitamin D, calcium and inflammatory biomarkers; clinical periodontal measurements; anthropometric measurements; and socio-demographic questionnaires are collected at baseline, third trimester and 6-8 weeks postpartum. Qualitative data are collected using focus group, for analysis of favourable factors and barriers related to study adherence.
Discussion: Oral health and mineral/vitamin supplementation are much overlooked in the public prenatal assistance in Brazil and of scarcity of clinical trials addressing these issues in low and middle-income countries,. To fill this gap the present study was designed to assess the feasibility of a RCT on acceptability of a multi-component intervention combining conventional periodontal treatment and consumption of milk fortified with calcium-vitamin D for improving periodontal conditions and maternal metabolic and inflammation status, among Brazilian low-income pregnant women with periodontitis. Thus, we hope that this relatively low-cost and safe multicomponent intervention can help reduce inflammation, improve maternal periodontal health and metabolic profile and consequently prevent negative gestational outcomes.
Trial registration: NCT, NCT03148483. Registered on May 11, 2017.
Keywords: Calcium; Feasibility randomised controlled trial; Milk; Periodontal therapy; Pregnant women; Vitamin D.
Conflict of interest statement
The study was presented to and approved by the director of the health centre where it is being conducted. Recruitment started after the IMPROVE trial protocol was submitted and approved by the Ethics Committee of School Maternity of the Federal University of Rio de Janeiro-Brazil on April 27, 2016 (certificate number 1.516.656). The study is being conducted according to the principles of Good Clinical Practice and the Declaration of Helsinki. Explanation of study procedures is given verbally to all participants and a written copy is provided in the patient information sheet. Study participation began after receipt of informed written consent. The trial was registered in the ClinicalTrials.gov database (NCT, NCT03148483. Registered 11 May 2017, https://clinicaltrials.gov/ct2/show/NCT03148483) and its recruitment began on May 2017.No applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Moynihan P, Bradbury J. Compromised dental function and nutrition. Nutrition. 2001;17(2):177–178. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous