Dynamic Control of the Self-Assembling Properties of Cyclodextrins by the Interplay of Aromatic and Host-Guest Interactions
- PMID: 30873399
- PMCID: PMC6401617
- DOI: 10.3389/fchem.2019.00072
Dynamic Control of the Self-Assembling Properties of Cyclodextrins by the Interplay of Aromatic and Host-Guest Interactions
Abstract
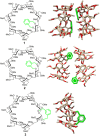
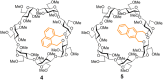
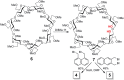
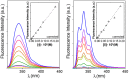
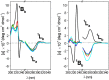
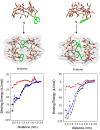
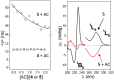
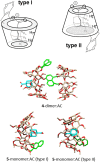
The presence of a doubly-linked naphthylene clip at the O-2I and O-3II positions in the secondary ring of β-cyclodextrin (βCD) derivatives promoted their self-assembly into head-to-head supramolecular dimers in which the aromatic modules act either as cavity extension walls (if the naphthalene moiety is 1,8-disubstituted) or as folding screens that separate the individual βCD units (if 2,3-disubstituted). Dimer architecture is governed by the conformational properties of the monomer constituents, as determined by NMR, fluorescence, circular dichroism, and computational techniques. In a second supramolecular organization level, the topology of the assembly directs host-guest interactions and, reciprocally, guest inclusion impacts the stability of the supramolecular edifice. Thus, inclusion of adamantane carboxylate, a well-known βCD cavity-fitting guest, was found to either preserve the dimeric arrangement, leading to multicomponent species, or elicit dimer disruption. The ensemble of results highlights the potential of the approach to program self-organization and external stimuli responsiveness of CD devices in a controlled manner while keeping full diastereomeric purity.
Keywords: circular dichroism; cyclodextrins; fluorescence; host-guest chemistry; naphthalene; self-assembly; supramolecular chemistry.
Figures

References
-
- Aoyagi T., Nakamura A., Ikeda H., Ikeda T., Mihara H., Ueno A. (1997). Alizarin Yellow-modified? β-cyclodextrin as a guest-responsive absorption change sensor. Anal. Chem. 69, 659–663. 10.1021/ac960727z - DOI
-
- Aranda C., Urbiola K., Méndez Ardoy A., García Fernández J. M., Ortiz Mellet C., Tros de Ilarduya C. (2013). Targeted gene delivery by new folate–polycationic amphiphilic cyclodextrin–DNA nanocomplexes in vitro and in vivo. Eur. J. Pharm. Bipharm. 85, 390–397. 10.1016/j.ejpb.2013.06.011 - DOI - PubMed
LinkOut - more resources
Full Text Sources

