New Applications of Synthetic Biology Tools for Cyanobacterial Metabolic Engineering
- PMID: 30873404
- PMCID: PMC6400836
- DOI: 10.3389/fbioe.2019.00033
New Applications of Synthetic Biology Tools for Cyanobacterial Metabolic Engineering
Abstract
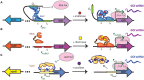
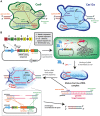
Cyanobacteria are promising microorganisms for sustainable biotechnologies, yet unlocking their potential requires radical re-engineering and application of cutting-edge synthetic biology techniques. In recent years, the available devices and strategies for modifying cyanobacteria have been increasing, including advances in the design of genetic promoters, ribosome binding sites, riboswitches, reporter proteins, modular vector systems, and markerless selection systems. Because of these new toolkits, cyanobacteria have been successfully engineered to express heterologous pathways for the production of a wide variety of valuable compounds. Cyanobacterial strains with the potential to be used in real-world applications will require the refinement of genetic circuits used to express the heterologous pathways and development of accurate models that predict how these pathways can be best integrated into the larger cellular metabolic network. Herein, we review advances that have been made to translate synthetic biology tools into cyanobacterial model organisms and summarize experimental and in silico strategies that have been employed to increase their bioproduction potential. Despite the advances in synthetic biology and metabolic engineering during the last years, it is clear that still further improvements are required if cyanobacteria are to be competitive with heterotrophic microorganisms for the bioproduction of added-value compounds.
Keywords: cyanobacteria; genome scale models; metabolic engineering; photosynthesis; synthetic biology.
Figures


References
-
- Acién F. G., Molina E., Reis A., Torzillo G., Zittelli G. C., Sepúlveda C., et al. (2017). Photobioreactors for the production of microalgae, in Microalgae-Based Biofuels and Bioproducts, eds Gonzalez-Fernandez C., Muñoz R. (Kidlington: Woodhead Publishing; ), 1–44.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

