Porcine Hemagglutinating Encephalomyelitis Virus: A Review
- PMID: 30873421
- PMCID: PMC6402421
- DOI: 10.3389/fvets.2019.00053
Porcine Hemagglutinating Encephalomyelitis Virus: A Review
Abstract
The porcine hemagglutinating encephalomyelitis virus (PHEV) is classified as a member of genus Betacoronavirus, family Coronaviridae, sub-family Cornavirinae, and order Nidovirales. PHEV shares the same genomic organization, replication strategy, and expression of viral proteins as other nidoviruses. PHEV produces vomiting and wasting disease (VWD) and/or encephalomyelitis, being the only known neurotropic coronavirus affecting pigs. First clinical outbreak was reported in 1957 in Ontario, Canada. Although pigs are the only species susceptible to natural PHEV infections, the virus displays neurotropism in mice and Wistar rats. Clinical disease, morbidity, and mortality is age-dependent and generally reported only in piglets under 4 weeks old. The primary site of replication of PHEV in pigs is the respiratory tract, and it can be further spread to the central nervous system through the peripheral nervous system via different pathways. The diagnosis of PHEV can be made using a combination of direct and indirect detection methods. The virus can be isolated from different tissues within the acute phase of the clinical signs using primary and secondary pig-derived cell lines. PHEV agglutinates the erythrocytes of mice, rats, chickens, and several other animals. PCR-based methods are useful to identify and subsequently isolate animals that are actively shedding the virus. The ability to detect antibodies allows producers to know the status of first-litter gilts and evaluate their risk of tier offspring to infection. PHEV is highly prevalent and circulates subclinically in most swine herds worldwide. PHEV-related disease is not clinically relevant in most of the swine-producing countries, most likely because of dams are immune to PHEV which may confer passive immunity to their offspring. However, PHEV should be considered a major source of economic loss because of the high mortality on farms with high gilt replacement rates, specific pathogen-free animals, and gnotobiotic swine herds. Thus, in the absence of current PHEV vaccines, promoting virus circulation on farms with early exposure to gilts and young sows could induce maternal immunity and prevent disease in piglets.
Keywords: coronavirus; encephalomyelitis; nidovirus; porcine hemagglutinating encephalomyelitis virus; vomiting and wasting disease.
Figures

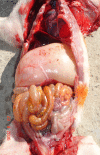
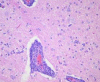
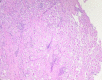
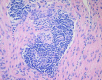
References
-
- Enjuanes L, van Der Zeijst BAM. Molecular basis of transmissible gastroenteritis virus epidemiology. In: Siddell SG. editor. The Coronaviridae The Viruses. Boston, MA: Springer; (1995). p. 337–76. 10.1007/978-1-4899-1531-3_16 - DOI
-
- Saif LJ, Pensaert M, Sestak K, Yeo S, Jung K. Hemagglutinating encephalomyelitis virus. In: Zimmerman JJ, Karriker L, Ramirez A, Schwartz KJ, Stevenson GW. editors. Diseases of swine. 10th ed Ames, IA: Blackwell Publishers; (2012). p. 517–20.
-
- Laude H, Van Reeth K, Pensaert M. Porcine respiratory coronavirus: molecular features and virus-host interactions. Vet Res. (1993) 24:125–50. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

