The physiology of artificial hibernation
- PMID: 30873448
- PMCID: PMC6410623
The physiology of artificial hibernation
Abstract
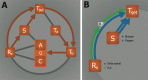
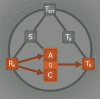
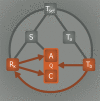
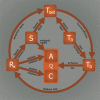
Incomplete understanding of the mechanisms responsible for induction of hibernation prevent translation of natural hibernation to its artificial counterpart. To facilitate this translation, a model was developed that identifies the necessary physiological changes for induction of artificial hibernation. This model encompasses six essential components: metabolism (anabolism and catabolism), body temperature, thermoneutral zone, substrate, ambient temperature, and hibernation-inducing agents. The individual components are interrelated and collectively govern the induction and sustenance of a hypometabolic state. To illustrate the potential validity of this model, various pharmacological agents (hibernation induction trigger, delta-opioid, hydrogen sulfide, 5'-adenosine monophosphate, thyronamine, 2-deoxyglucose, magnesium) are described in terms of their influence on specific components of the model and corollary effects on metabolism. Relevance for patients: The ultimate purpose of this model is to help expand the paradigm regarding the mechanisms of hibernation from a physiological perspective and to assist in translating this natural phenomenon to the clinical setting.
Keywords: Arrhenius law; anapyrexia; body temperature; hypometabolic agents; hypometabolism; hypoxia; natural hibernation; thermal convection; thermoneutral zone; torpor.
Conflict of interest statement
The authors declare that there are no conflicts of interest present.
Figures


Similar articles
-
Natural hypometabolism during hibernation and daily torpor in mammals.Respir Physiol Neurobiol. 2004 Aug 12;141(3):317-29. doi: 10.1016/j.resp.2004.03.014. Respir Physiol Neurobiol. 2004. PMID: 15288602 Review.
-
Induction of torpor: mimicking natural metabolic suppression for biomedical applications.J Cell Physiol. 2012 Apr;227(4):1285-90. doi: 10.1002/jcp.22850. J Cell Physiol. 2012. PMID: 21618525 Review.
-
Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature.Physiol Rev. 2003 Oct;83(4):1153-81. doi: 10.1152/physrev.00008.2003. Physiol Rev. 2003. PMID: 14506303 Review.
-
Strategies of biochemical adaptation for hibernation in a South American marsupial, Dromiciops gliroides: 2. Control of the Akt pathway and protein translation machinery.Comp Biochem Physiol B Biochem Mol Biol. 2018 Oct;224:19-25. doi: 10.1016/j.cbpb.2017.12.006. Epub 2017 Dec 13. Comp Biochem Physiol B Biochem Mol Biol. 2018. PMID: 29247846
-
Antipsychotic inductors of brain hypothermia and torpor-like states: perspectives of application.Psychopharmacology (Berl). 2017 Jan;234(2):173-184. doi: 10.1007/s00213-016-4496-2. Epub 2016 Dec 8. Psychopharmacology (Berl). 2017. PMID: 27933367 Review.
Cited by
-
Pathophysiology of Hemorrhage as It Relates to the Warfighter.Physiology (Bethesda). 2022 May 1;37(3):141-153. doi: 10.1152/physiol.00028.2021. Epub 2022 Jan 10. Physiology (Bethesda). 2022. PMID: 35001653 Free PMC article. Review.
-
Carotid chemoreceptor denervation does not impair hypoxia-induced thermal downregulation but vitiates recovery from a hypothermic and hypometabolic state in mice.Sci Rep. 2019 Mar 26;9(1):5132. doi: 10.1038/s41598-019-41546-x. Sci Rep. 2019. PMID: 30914789 Free PMC article.
References
-
- Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–274. - PubMed
-
- Hudson JW, Scott IM. Daily torpor in the laboratory mouse, Musmusculus var. albino. Physiological Zoology. 1979;52:205–218.
-
- Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. - PubMed
-
- Dark J, Miller DR, Zucker I. Reduced glucose availability induces torpor in Siberian hamsters. Am J Physiol. 1994;267:R496–R501. - PubMed
-
- Horton ND, Kaftani DJ, Bruce DS, Bailey EC, Krober AS, Jones JR, Turker M, Khattar N, Su TP, Bolling SF, Oeltgen PR. Isolation and partial characterization of an opioid-like 88 kDa hibernation-related protein. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:787–805. - PubMed
LinkOut - more resources
Full Text Sources
