Extrafollicular responses in humans and SLE
- PMID: 30874345
- PMCID: PMC6422038
- DOI: 10.1111/imr.12741
Extrafollicular responses in humans and SLE
Abstract
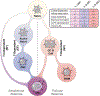
Chronic autoimmune diseases, and in particular Systemic Lupus Erythematosus (SLE), are endowed with a long-standing autoreactive B-cell compartment that is presumed to reactivate periodically leading to the generation of new bursts of pathogenic antibody-secreting cells (ASC). Moreover, pathogenic autoantibodies are typically characterized by a high load of somatic hypermutation and in some cases are highly stable even in the context of prolonged B-cell depletion. Long-lived, highly mutated antibodies are typically generated through T-cell-dependent germinal center (GC) reactions. Accordingly, an important role for GC reactions in the generation of pathogenic autoreactivity has been postulated in SLE. Nevertheless, pathogenic autoantibodies and autoimmune disease can be generated through B-cell extrafollicular (EF) reactions in multiple mouse models and human SLE flares are characterized by the expansion of naive-derived activated effector B cells of extrafollicular phenotype. In this review, we will discuss the properties of the EF B-cell pathway, its relationship to other effector B-cell populations, its role in autoimmune diseases, and its contribution to human SLE. Furthermore, we discuss the relationship of EF B cells with Age-Associated B cells (ABCs), a TLR-7-driven B-cell population that mediates murine autoimmune and antiviral responses.
Keywords: ABC; B cells; DN2; SLE; extrafollicular; plasma cells.
© 2019 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest Statement
The authors declare no conflicts of interest.
Figures

References
-
- Katakai T, Suto H, Sugai M, et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol 2008;181:6189–6200. - PubMed
-
- Gonzalez SF, Degn SE, Pitcher LA, Woodruff M, Heesters BA, Carroll MC. Trafficking of B cell antigen in lymph nodes. Annu Rev Immunol 2011;29:215–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

