Restoration of high-sensitivity and adapting vision with a cone opsin
- PMID: 30874546
- PMCID: PMC6420663
- DOI: 10.1038/s41467-019-09124-x
Restoration of high-sensitivity and adapting vision with a cone opsin
Abstract
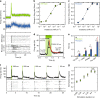
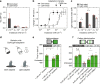
Inherited and age-related retinal degenerative diseases cause progressive loss of rod and cone photoreceptors, leading to blindness, but spare downstream retinal neurons, which can be targeted for optogenetic therapy. However, optogenetic approaches have been limited by either low light sensitivity or slow kinetics, and lack adaptation to changes in ambient light, and not been shown to restore object vision. We find that the vertebrate medium wavelength cone opsin (MW-opsin) overcomes these limitations and supports vision in dim light. MW-opsin enables an otherwise blind retinitis pigmenotosa mouse to discriminate temporal and spatial light patterns displayed on a standard LCD computer tablet, displays adaption to changes in ambient light, and restores open-field novel object exploration under incidental room light. By contrast, rhodopsin, which is similar in sensitivity but slower in light response and has greater rundown, fails these tests. Thus, MW-opsin provides the speed, sensitivity and adaptation needed to restore patterned vision.
Conflict of interest statement
J.G.F and E.Y.I. are founders of Photoswitch Therapeutics, a startup whose goal is to restore vision in blinding disease using photo-pharmacology, chemical optogenetics, and optogenetic methods of the kind described here. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

