Life habits and evolutionary biology of new two-winged long-proboscid scorpionflies from mid-Cretaceous Myanmar amber
- PMID: 30874563
- PMCID: PMC6420582
- DOI: 10.1038/s41467-019-09236-4
Life habits and evolutionary biology of new two-winged long-proboscid scorpionflies from mid-Cretaceous Myanmar amber
Abstract
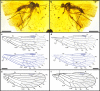
Long-proboscid scorpionflies are enigmatic, mid-Mesozoic insects associated with gymnosperm pollination. One major lineage, Aneuretopsychina, consists of four families plus two haustellate clades, Diptera and Siphonaptera. One clade, Pseudopolycentropodidae, from mid-Cretaceous Myanmar amber, contains Parapolycentropus. Here, we newly establish Dualula, assigned to Dualulidae, constituting the fifth lineage. Parapolycentropus and Dualula lineages are small, two-winged, with unique siphonate mouthparts for imbibing pollination drops. A cibarial pump provides siphonal food inflow; in Dualula, the siphon base surrounds a hypopharynx housing a small, valved pump constricted to a narrow salivary duct supplying outgoing enzymes for food fluidization. Indirect evidence links long-proboscid mouthpart structure with contemporaneous tubulate ovulate organs. Direct evidence of gymnospermous Cycadopites pollen is associated with one Parapolycentropus specimen. Parapolycentropus and Dualula exhibit hind-wing reduction that would precede haltere formation, likely caused by Ultrabithorax. Distinctive, male Aneuretopsychina genitalia are evident from specimens in copulo, supplemented by mixed-sex individuals of likely male mating swarms.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Labandeira CC. The pollination of mid Mesozoic seed plants and the early history of long-proboscid insects. Ann. Mo. Bot. Gard. 2010;97(4):469–513. doi: 10.3417/2010037. - DOI
-
- Bashkuev AS. Nedubroviidae, a new family of Mecoptera: the first Paleozoic long-proboscid scorpionflies. Zootaxa. 2011;2895(1):47–57. doi: 10.11646/zootaxa.2895.1.3. - DOI
-
- Grimaldi DA, Engel MS. Evolution of the Insects. Cambridge: Cambridge University Press; 2005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

