Controlling a Chemical Coupling Reaction on a Surface: Tools and Strategies for On-Surface Synthesis
- PMID: 30875199
- PMCID: PMC6477809
- DOI: 10.1021/acs.chemrev.8b00601
Controlling a Chemical Coupling Reaction on a Surface: Tools and Strategies for On-Surface Synthesis
Abstract
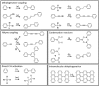
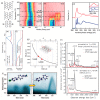
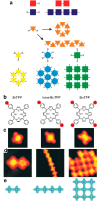
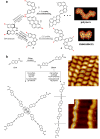
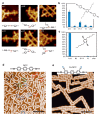
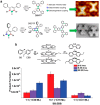
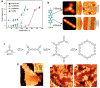
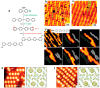
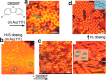
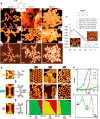
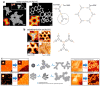
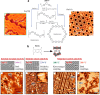
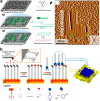
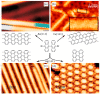
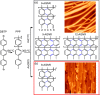
On-surface synthesis is appearing as an extremely promising research field aimed at creating new organic materials. A large number of chemical reactions have been successfully demonstrated to take place directly on surfaces through unusual reaction mechanisms. In some cases the reaction conditions can be properly tuned to steer the formation of the reaction products. It is thus possible to control the initiation step of the reaction and its degree of advancement (the kinetics, the reaction yield); the nature of the reaction products (selectivity control, particularly in the case of competing processes); as well as the structure, position, and orientation of the covalent compounds, or the quality of the as-formed networks in terms of order and extension. The aim of our review is thus to provide an extensive description of all tools and strategies reported to date and to put them into perspective. We specifically define the different approaches available and group them into a few general categories. In the last part, we demonstrate the effective maturation of the on-surface synthesis field by reporting systems that are getting closer to application-relevant levels thanks to the use of advanced control strategies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




















References
-
- Shen Q.; Gao H. Y.; Fuchs H. Frontiers of On-Surface Synthesis: From Principles to Applications. Nano Today 2017, 13, 77–96. 10.1016/j.nantod.2017.02.007. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

