Molecular Mechanisms of Hypothalamic Insulin Resistance
- PMID: 30875909
- PMCID: PMC6471380
- DOI: 10.3390/ijms20061317
Molecular Mechanisms of Hypothalamic Insulin Resistance
Abstract
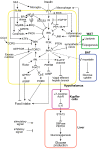
Insulin exists in the central nervous system, where it executes two important functions in the hypothalamus: the suppression of food intake and the improvement of glucose metabolism. Recent studies have shown that both are exerted robustly in rodents and humans. If intact, these functions exert beneficial effects on obesity and diabetes, respectively. Disruption of both occurs due to a condition known as hypothalamic insulin resistance, which is caused by obesity and the overconsumption of saturated fat. An enormous volume of literature addresses the molecular mechanisms of hypothalamic insulin resistance. IKKβ and JNK are major players in the inflammation pathway, which is activated by saturated fatty acids that induce hypothalamic insulin resistance. Two major tyrosine phosphatases, PTP-1B and TCPTP, are upregulated in chronic overeating. They dephosphorylate the insulin receptor and insulin receptor substrate proteins, resulting in hypothalamic insulin resistance. Prolonged hyperinsulinemia with excessive nutrition activates the mTOR/S6 kinase pathway, thereby enhancing IRS-1 serine phosphorylation to induce hypothalamic insulin resistance. Other mechanisms associated with this condition include hypothalamic gliosis and disturbed insulin transport into the central nervous system. Unveiling the precise molecular mechanisms involved in hypothalamic insulin resistance is important for developing new ways of treating obesity and type 2 diabetes.
Keywords: food intake; glucose metabolism; hypothalamus; inflammation; insulin resistance; obesity.
Conflict of interest statement
The author has no conflicts of interest to declare.
Figures
References
-
- Kimura K., Tanida M., Nagata N., Inaba Y., Watanabe H., Nagashimada M., Ota T., Asahara S., Kido Y., Matsumoto M., et al. Central Insulin Action Activates Kupffer Cells by Suppressing Hepatic Vagal Activation via the Nicotinic Alpha 7 Acetylcholine Receptor. Cell Rep. 2016;14:2362–2374. doi: 10.1016/j.celrep.2016.02.032. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

