Noninvasive high-frequency oscillatory ventilation as respiratory support in preterm infants: a meta-analysis of randomized controlled trials
- PMID: 30876411
- PMCID: PMC6420773
- DOI: 10.1186/s12931-019-1023-0
Noninvasive high-frequency oscillatory ventilation as respiratory support in preterm infants: a meta-analysis of randomized controlled trials
Abstract
Background: Noninvasive high-frequency oscillatory ventilation (nHFOV), a relatively new modality, is gaining popularity despite scarce evidence. This meta-analysis was designed to evaluate the efficacy and safety of nHFOV as respiratory support in premature infants.
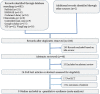
Methods: We searched MEDLINE, EMBASE, CINAHL, and Cochrane CENTRAL from inception of the database to January 2019. All published randomized controlled trials (RCTs) evaluating the effect of nHFOV therapy with nasal continuous positive airway pressure (nCPAP) or biphasic nCPAP (BP-CPAP) in newborns for respiratory support were included. All meta-analyses were performed using Review Manager 5.3.
Results: A total of 8 RCTs involving 463 patients were included. The meta-analysis estimated a lower risk of intubation (relative risk = 0.50, 95% confidence interval of 0.36 to 0.70) and more effective clearance of carbon dioxide (weighted mean difference = - 4.61, 95% confidence interval of - 7.94 to - 1.28) in the nHFOV group than in the nCPAP/BP-CPAP group.
Conclusions: Our meta-analysis of RCTs suggests that nHFOV, as respiratory support in preterm infants, significantly remove carbon dioxide and reduce the risk of intubation compared with nCPAP/BP-CPAP. The appropriate parameter settings for different types of noninvasive high-frequency ventilators, the effect of nHFOV in extremely preterm infants, and the long-term safety of nHFOV need to be assessed in large trials.
Keywords: Bronchopulmonary dysplasia; Continuous positive airway pressure; Noninvasive high-frequency oscillatory ventilation; Preterm infants.
Conflict of interest statement
Ethics approval and consent to participate
As the paper did not involve any human or animal, the ethical approval was not required.
Competing interests
None of the investigators declare any real or perceived conflicts of interest pertaining to the subject of this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S. Eunice Kennedy Shriver National Institute of Child Health and Human Development neonatal research network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. - DOI - PMC - PubMed
-
- Cummings JJ, Polin RA. AAP the committee on fetus and newborn. Noninvasive Respir Support Pediatr. 2016;137:e20153758.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical