The clinical course of idiopathic pulmonary fibrosis and its association to quality of life over time: longitudinal data from the INSIGHTS-IPF registry
- PMID: 30876420
- PMCID: PMC6420774
- DOI: 10.1186/s12931-019-1020-3
The clinical course of idiopathic pulmonary fibrosis and its association to quality of life over time: longitudinal data from the INSIGHTS-IPF registry
Abstract
Background: Quality of life (QoL) is profoundly impaired in patients with idiopathic pulmonary fibrosis (IPF). However, data is limited regarding the course of QoL. We therefore analysed longitudinal data from the German INSIGHTS-IPF registry.
Methods: Clinical status and QoL were assessed at enrollment and subsequently at 6- to 12-months intervals. A range of different QoL questionnaires including the St. George's Respiratory Questionnaire (SGRQ) were used.
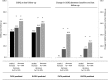
Results: Data from 424 patients were included; 76.9% male; mean age 68.7 ± 9.1 years, mean FVC% predicted 75.9 ± 19.4, mean DLCO% predicted 36.1 ± 15.9. QoL worsened significantly during follow-up with higher total SGRQ scores (increased by 1.47 per year; 95% CI: 1.17 to 1.76; p < 0.001) and higher UCSD-SOBQ scores and lower EQ-5D VAS and WHO-5 scores. An absolute decline in FVC% predicted of > 10% was associated with a significant deterioration in SGRQ (increasing by 9.08 units; 95% CI: 2.48 to 15.67; p = 0.007), while patients with stable or improved FVC had no significantly change in SGRQ. Patients with a > 10% decrease of DLCO % predicted also had a significant increase in SGRQ (+ 7.79 units; 95% CI: 0.85 to 14.73; p = 0.028), while SQRQ was almost stable in patients with stable or improved DLCO. Patients who died had a significant greater increase in SGRQ total scores (mean 11.8 ± 18.6) at their last follow-up visit prior to death compared to survivors (mean 4.2 ± 18.9; HR = 1.03; 95% CI: 1.01 to 1.04; p < 0.001). All QoL scores across the follow-up period were significantly worse in hospitalised patients compared to non-hospitalised patients, with the worst scores reported in those hospitalised for acute exacerbations.
Conclusions: QoL assessments in the INSIGHTS-IPF registry demonstrate a close relationship between QoL and clinically meaningful changes in lung function, comorbidities, disease duration and clinical course of IPF, including hospitalisation and mortality.
Keywords: Cohort study; Idiopathic pulmonary fibrosis; Patient-related outcomes; SQRQ.
Conflict of interest statement
Ethics approval and consent to participate
The study materials were approved by the Ethics Committee of the Medical Faculty, Technical University of Dresden (EK 255082012), and by further local ethic committees as per local requirements.
Consent for publication
Not applicable.
Competing interests
MK reports grants and personal fees from Roche/InterMune, grants and personal fees from Boehringer Ingelheim, outside the submitted work; AP reports grants and personal fees from Roche/InterMune, grants and personal fees from Boehringer Ingelheim, outside the submitted work; HuWi reports personal fees from Boehringer Ingelheim, and personal fees from Roche, outside the submitted work; MC reports honoraria for lectures from Boehringer Ingelheim Pharma GmbH and Roche Pharma, and for serving on advisory boards from Boehringer Ingelheim, outside the submitted work; DP reports personal fees outside the submitted work from Actelion, Bayer, Boehringer Ingelheim, Sanofi, Biogen, Shield and MSD; DS reports personal fees from Boehringer Ingelheim, Roche, outside the submitted work; SV reports personal fees from Boehringer Ingelheim, personal fees from Roche Pharma, personal fees from Actelion Pharma, grants and personal fees from Novartis Pharma, personal fees from Berlin Chemie, and personal fees from Astra, outside the submitted work; HeWi reports personal fees from Boehringer, personal fees from Roche, during the conduct of the study; personal fees from Bayer, personal fees from Biotest, personal fees from Actelion, personal fees from GSK, and personal fees from Pfizer, outside the submitted work; CN reports honoraria for lectures and serving on advisory boards from Boehringer Ingelheim and Roche Pharma; SA reports case payments from Boehringer Ingelheim, during the conduct of the study; personal fees from Boehringer Ingelheim, and personal fees from Roche, outside the submitted work; SG reports personal fees from Boehringer Ingelheim, personal fees from Roche Pharma, personal fees from Actelion Pharma, grants and personal fees from Novartis Pharma, personal fees from Berlin Chemie, and personal fees from Astra, all outside the submitted work; TW reports grants from Boehringer, during the conduct of the study; TB reports grants from German Center for Lung Research (DZL), personal fees for consultation or lecture from Roche, AstraZeneca, Chiesi, GSK, and Novartis outside the submitted work; JB received grants from Boehringer Ingelheim, and personal fees for consultation or lectures from Actelion, Bayer, Boehringer-Ingelheim, and Roche. He is member of the national and international IPF guideline committee; All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

