Comparative Risk of Serious Infections With Biologic and/or Immunosuppressive Therapy in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis
- PMID: 30876964
- PMCID: PMC8011651
- DOI: 10.1016/j.cgh.2019.02.044
Comparative Risk of Serious Infections With Biologic and/or Immunosuppressive Therapy in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis
Abstract
Background & aims: We performed a systematic review and meta-analysis to evaluate the comparative risk of serious infections with tumor necrosis factor (TNF) antagonists, non-TNF targeted biologics, tofacitinib, and immunosuppressive agents in patients with inflammatory bowel diseases (IBDs).
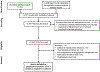
Methods: In a systematic search of publications, through March 18, 2018, we identified 15 observational studies (>500 person-years) of patients with IBD treated with TNF antagonists, non-TNF targeted biologics, tofacitinib, and/or immunosuppressive agents (thiopurines, methotrexate) that reported risk of serious infections. Only studies with active comparators were included, to allow appropriate comparative synthesis. We performed random-effects meta-analysis and estimated relative risk (RR) and 95% CIs.
Results: Compared with anti-TNF monotherapy, risk of serious infection increased with the combination of anti-TNF and an immunosuppressive agent (in 6 cohorts: RR, 1.19; 95% CI, 1.03-1.37), with anti-TNF and a corticosteroid (in 4 cohorts: RR, 1.64; 95% CI, 1.33-2.03), or with all 3 drugs (in 2 cohorts: RR, 1.35; 95% CI, 1.04-1.77); there was minimal heterogeneity among studies. In contrast, monotherapy with an immunosuppressive agent was associated with a lower risk of serious infections than monotherapy with a TNF antagonist (7 cohorts: RR, 0.61; 95% CI 0.44-0.84) or a TNF antagonist with an immunosuppressive agent (2 cohorts: RR, 0.56; 95% CI, 0.39-0.81). Infliximab-based therapy was associated with a lower risk of serious infections compared with adalimumab-based therapy in patients with ulcerative colitis (4 cohorts: RR, 0.57; 95% CI, 0.33-0.97), but not Crohn's disease (4 cohorts: RR, 0.91; 95% CI, 0.49-1.70). Few data were available on the comparative safety of biologic agents that do not inhibit TNF and tofacitinib.
Conclusions: Combination therapies for IBD that include TNF antagonists, especially with corticosteroids, are associated with a higher risk of serious infection, whereas monotherapy with an immunosuppressive agent is associated with a lower risk, compared with monotherapy with a TNF antagonist. Studies are needed to evaluate the comparative safety of non-TNF targeted biologics and small molecules for treatment of IBD.
Keywords: CD; Crohn’s Disease; UC; Ustekinumab; Vedolizumab.
Copyright © 2020 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Nguyen NH, Ohno-Machado L, Sandborn WJ, et al. Infections and Cardiovascular Complications are Common Causes for Hospitalization in Older Patients with Inflammatory Bowel Diseases. Inflamm Bowel Dis 2018;24:916–923. - PubMed
-
- Osterman MT, Sandborn WJ, Colombel JF, et al. Crohn’s Disease Activity and Concomitant Immunosuppressants Affect the Risk of Serious and Opportunistic Infections in Patients Treated With Adalimumab. American Journal of Gastroenterology 2016;111:1806–1815. - PubMed
-
- Targownik LE, Bernstein CN. Infectious and malignant complications of TNF inhibitor therapy in IBD. Am J Gastroenterol 2013;108:1835–42, quiz 1843. - PubMed

