Limiting tumor seeding as a therapeutic approach for metastatic disease
- PMID: 30877019
- PMCID: PMC6571062
- DOI: 10.1016/j.pharmthera.2019.03.007
Limiting tumor seeding as a therapeutic approach for metastatic disease
Abstract
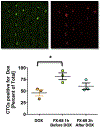
Here we propose that therapeutic targeting of circulating tumor cells (CTCs), which are widely understood to be the seeds of metastasis, would represent an effective strategy towards limiting numerical expansion of secondary lesions and containing overall tumor burden in cancer patients. However, the molecular mediators of tumor seeding have not been well characterized. This is in part due to the limited number of pre-clinical in vivo approaches that appropriately interrogate the mechanisms by which cancer cells home to arresting organs. It is critical that we continue to investigate the mediators of tumor seeding as it is evident that the ability of CTCs to colonize in distant sites is what drives disease progression even after the primary tumor has been ablated by local modalities. In addition to slowing disease progression, containing metastatic spread by impeding tumor cell seeding may also provide a clinical benefit by increasing the duration of the residence of CTCs in systemic circulation thereby increasing their exposure to pharmacological agents commonly used in the treatment of patients such as chemotherapy and immunotherapies. In this review we will examine the current state of knowledge about the mechanisms of tumor cells seeding as well as explore how targeting this stage of metastatic spreading may provide therapeutic benefit to patients with advanced disease.
Keywords: CX3CR1; Chemokines; Integrins; Metastasis; Seeding; Selectins.
Copyright © 2019. Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

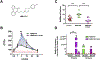
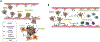
References
-
- Albelda SM (1993). Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Laboratory Investigation 68(1), 4–17. - PubMed
-
- Anantharaman A, Friedlander T, Lu D, Krupa R, Premasekharan G, Hough J, et al. (2016). Programmed death-ligand 1 (PD-L1) characterization of circulating tumor cells (CTCs) in muscle invasive and metastatic bladder cancer patients. BMC Cancer 16(1), 289 10.1186/s12885-016-2758-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

