Crystal structure of the TreS:Pep2 complex, initiating α-glucan synthesis in the GlgE pathway of mycobacteria
- PMID: 30877199
- PMCID: PMC6509496
- DOI: 10.1074/jbc.RA118.004297
Crystal structure of the TreS:Pep2 complex, initiating α-glucan synthesis in the GlgE pathway of mycobacteria
Abstract
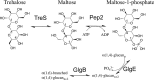
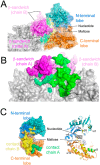
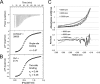
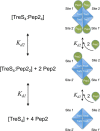
A growing body of evidence implicates the mycobacterial capsule, the outermost layer of the mycobacterial cell envelope, in modulation of the host immune response and virulence of mycobacteria. Mycobacteria synthesize the dominant capsule component, α(1→4)-linked glucan, via three interconnected and potentially redundant metabolic pathways. Here, we report the crystal structure of the Mycobacterium smegmatis TreS:Pep2 complex, containing trehalose synthase (TreS) and maltokinase (Pep2), which converts trehalose to maltose 1-phosphate as part of the TreS:Pep2-GlgE pathway. The structure, at 3.6 Å resolution, revealed that a diamond-shaped TreS tetramer forms the core of the complex and that pairs of Pep2 monomers bind to opposite apices of the tetramer in a 4 + 4 configuration. However, for the M. smegmatis orthologues, results from isothermal titration calorimetry and analytical ultracentrifugation experiments indicated that the prevalent stoichiometry in solution is 4 TreS + 2 Pep2 protomers. The observed discrepancy between the crystallized complex and the behavior in the solution state may be explained by the relatively weak affinity of Pep2 for TreS (Kd 3.5 μm at mildly acidic pH) and crystal packing favoring the 4 + 4 complex. Proximity of the ATP-binding site in Pep2 to the complex interface provides a rational basis for rate enhancement of Pep2 upon binding to TreS, but the complex structure appears to rule out substrate channeling between the active sites of TreS and Pep2. Our findings provide a structural model for the trehalose synthase:maltokinase complex in M. smegmatis that offers critical insights into capsule assembly.
Keywords: X-ray crystallography; analytical ultracentrifugation; capsule; enzyme catalysis; immune evasion; maltose kinase; mycobacteria; pathogenesis; protein complex; trehalose; trehalose synthase; α-glucan.
© 2019 Kermani et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
-
- Mishra A. K., Driessen N. N., Appelmelk B. J., and Besra G. S. (2011) Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host–pathogen interaction. FEMS Microbiol. Rev. 35, 1126–1157 10.1111/j.1574-6976.2011.00276.x - DOI - PMC - PubMed
-
- Stokes R. W., Norris-Jones R., Brooks D. E., Beveridge T. J., Doxsee D., and Thorson L. M. (2004) The glycan-rich outer layer of the cell wall of Mycobacterium tuberculosis acts as an antiphagocytic capsule limiting the association of the bacterium with macrophages. Infect. Immun. 72, 5676–5686 10.1128/IAI.72.10.5676-5686.2004 - DOI - PMC - PubMed
-
- Geurtsen J., Chedammi S., Mesters J., Cot M., Driessen N. N., Sambou T., Kakutani R., Ummels R., Maaskant J., Takata H., Baba O., Terashima T., Bovin N., Vandenbroucke-Grauls C., Nigou J., et al. (2009) Identification of mycobacterial α-glucan as a novel ligand for DC-SIGN: involvement of mycobacterial capsular polysaccharides in host immune modulation. J. Immunol. 183, 5221–5231 10.4049/jimmunol.0900768 - DOI - PubMed
-
- Sambou T., Dinadayala P., Stadthagen G., Barilone N., Bordat Y., Constant P., Levillain F., Neyrolles O., Gicquel B., Lemassu A., Daffé M., and Jackson M. (2008) Capsular glucan and intracellular glycogen of Mycobacterium tuberculosis: biosynthesis and impact on the persistence in mice. Mol. Microbiol. 70, 762–774 10.1111/j.1365-2958.2008.06445.x - DOI - PMC - PubMed
-
- Romero M. M., Basile J. I., López B., Ritacco V., Barrera L., Sasiain Mdel C., and Alemán M. (2014) Outbreaks of Mycobacterium tuberculosis MDR strains differentially induce neutrophil respiratory burst involving lipid rafts, p38 MAPK and Syk. BMC Infect. Dis. 14, 262 10.1186/1471-2334-14-262 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

