Effect of Treatment on Imaging, Clinical, and Serologic Assessments of Disease Activity in Large-vessel Vasculitis
- PMID: 30877209
- PMCID: PMC12276863
- DOI: 10.3899/jrheum.181222
Effect of Treatment on Imaging, Clinical, and Serologic Assessments of Disease Activity in Large-vessel Vasculitis
Abstract
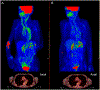
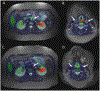
Objective: Disease activity in large-vessel vasculitis (LVV) is traditionally assessed by clinical and serological variables rather than vascular imaging. This study determined the effect of treatment on 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) vascular activity in relation to clinical- and serologic-based assessments.
Methods: Patients with giant cell arteritis (GCA) or Takayasu arteritis (TA) were prospectively evaluated at 6-month intervals in an observational cohort. Treatment changes were made at least 3 months before the followup visit and categorized as increased, decreased, or unchanged. Imaging (FDG-PET qualitative analysis), clinical, and serologic (erythrocyte sedimentation rate, C-reactive protein) assessments were determined at each visit and compared over interval visits.
Results: Serial assessments were performed in 52 patients with LVV (GCA = 31; TA = 21) over 156 visits. Increased, decreased, or unchanged therapy was recorded for 36-, 23-, and 32-visit intervals, respectively. When treatment was increased, there was significant reduction in disease activity by imaging, clinical, and inflammatory markers (p ≤ 0.01 for each). When treatment was unchanged, all 3 assessments of disease activity remained similarly unchanged over 6-month intervals. When treatment was reduced, PET activity significantly worsened (p = 0.02) but clinical and serologic activity did not significantly change. Treatment of GCA with tocilizumab and of TA with tumor necrosis factor inhibitors resulted in significant improvement in imaging and clinical assessments of disease activity, but only rarely did the assessments both become normal.
Conclusion: In addition to clinical and serologic assessments, vascular imaging has potential to monitor disease activity in LVV and should be tested as an outcome measure in randomized clinical trials.
Keywords: FLUORODEOXYGLUCOSE; GIANT CELL ARTERITIS; LARGE-VESSEL VASCULITIS; POSITRON EMISSION TOMOGRAPHY; TAKAYASU ARTERITIS; VASCULITIS.
Conflict of interest statement
Figures

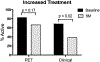
Similar articles
-
Management of large-vessel vasculitis with FDG-PET: a systematic literature review and meta-analysis.Medicine (Baltimore). 2015 Apr;94(14):e622. doi: 10.1097/MD.0000000000000622. Medicine (Baltimore). 2015. PMID: 25860208 Free PMC article.
-
Diagnostic value of [18F]FDG-PET/CT for treatment monitoring in large vessel vasculitis: a systematic review and meta-analysis.Eur J Nucl Med Mol Imaging. 2021 Nov;48(12):3886-3902. doi: 10.1007/s00259-021-05362-8. Epub 2021 May 3. Eur J Nucl Med Mol Imaging. 2021. PMID: 33942141 Free PMC article.
-
Longitudinal Characterization of Vascular Inflammation and Disease Activity in Takayasu Arteritis and Giant Cell Arteritis: A Single-Center Prospective Study.Arthritis Care Res (Hoboken). 2023 Jun;75(6):1362-1370. doi: 10.1002/acr.24976. Epub 2023 Jan 5. Arthritis Care Res (Hoboken). 2023. PMID: 35762866 Free PMC article.
-
Clinical symptoms and associated vascular imaging findings in Takayasu's arteritis compared to giant cell arteritis.Ann Rheum Dis. 2020 Feb;79(2):262-267. doi: 10.1136/annrheumdis-2019-216145. Epub 2019 Oct 24. Ann Rheum Dis. 2020. PMID: 31649025 Free PMC article.
-
Serial assessment of ultrasound sensitivity and scores in patients with giant cell arteritis before and 3 and 10 days after treatment.Rheumatology (Oxford). 2025 Jun 1;64(6):3895-3899. doi: 10.1093/rheumatology/keae551. Rheumatology (Oxford). 2025. PMID: 39400596
Cited by
-
Outcome Measures and Biomarkers for Disease Assessment in Takayasu Arteritis.Diagnostics (Basel). 2022 Oct 21;12(10):2565. doi: 10.3390/diagnostics12102565. Diagnostics (Basel). 2022. PMID: 36292253 Free PMC article. Review.
-
Disease-modifying anti-rheumatic drugs for the management of Takayasu arteritis-a systematic review and meta-analysis.Clin Rheumatol. 2021 Nov;40(11):4391-4416. doi: 10.1007/s10067-021-05743-2. Epub 2021 May 1. Clin Rheumatol. 2021. PMID: 33932173 Free PMC article.
-
Monitoring and long-term management of giant cell arteritis and polymyalgia rheumatica.Nat Rev Rheumatol. 2020 Sep;16(9):481-495. doi: 10.1038/s41584-020-0458-5. Epub 2020 Aug 5. Nat Rev Rheumatol. 2020. PMID: 32759996 Review.
-
Vascular Uptake on 18F-FDG PET/CT During the Clinically Inactive State of Takayasu Arteritis Is Associated with a Higher Risk of Relapse.Yonsei Med J. 2021 Sep;62(9):814-821. doi: 10.3349/ymj.2021.62.9.814. Yonsei Med J. 2021. PMID: 34427067 Free PMC article.
-
Measuring treatment outcomes and change in disease activity in giant cell arteritis: a systematic literature review informing the development of the EULAR-ACR response criteria on behalf of the EULAR-ACR response criteria in giant cell arteritis task force.RMD Open. 2023 Jun;9(2):e003233. doi: 10.1136/rmdopen-2023-003233. RMD Open. 2023. PMID: 37349123 Free PMC article.
References
-
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. - PubMed
-
- Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med. 1994;120(11):919–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
