Immune-Checkpoint-Inhibitor-Induced Severe Autoimmune Encephalitis Treated by Steroid and Intravenous Immunoglobulin
- PMID: 30877699
- PMCID: PMC6444136
- DOI: 10.3988/jcn.2019.15.2.259
Immune-Checkpoint-Inhibitor-Induced Severe Autoimmune Encephalitis Treated by Steroid and Intravenous Immunoglobulin
Conflict of interest statement
The authors have no financial conflicts of interest.
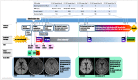
Figures
References
-
- Krishnamurthy A, Jimeno A. Atezolizumab: a novel PD-L1 inhibitor in cancer therapy with a focus in bladder and non-small cell lung cancers. Drugs Today (Barc) 2017;53:217–237. - PubMed
-
- Fellner A, Makranz C, Lotem M, Bokstein F, Taliansky A, Rosenberg S, et al. Neurologic complications of immune checkpoint inhibitors. J Neurooncol. 2018;137:601–609. - PubMed
-
- Food and Drug Administration. TECENTRIQ® (atezolizumab) injection, for intravenous use [Internet] USA: Silver Spring: Food and Drug Administration; 2018. [cited 2018 Sep 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761034s009lbl.pdf.
-
- Blackmon JT, Viator TM, Conry RM. Central nervous system toxicities of anti-cancer immune checkpoint blockade. J Neurol Neuromed. 2016;1:39–45.
-
- Ito M, Fujiwara S, Fujimoto D, Mori R, Yoshimura H, Hata A, et al. Rituximab for nivolumab plus ipilimumab-induced encephalitis in a small-cell lung cancer patient. Ann Oncol. 2017;28:2318–2319. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

