Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release
- PMID: 30878596
- PMCID: PMC6491392
- DOI: 10.1016/j.ebiom.2019.03.018
Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release
Abstract
Background: Treatment multiple tumors by immune therapy can be achieved by mobilizing both innate and adaptive immunity. The programmed death ligand 1 (PD-L1; or CD274, B7-H1) is a critical "don't find me" signal to the adaptive immune system. Equally CD47 is a critical "don't eat me" signal to the innate immune system and a regulator of the adaptive immune response.
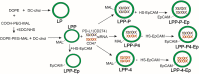
Method: Both of CD47 and PD-L1 are overexpressed on the surface of cancer cells to enable to escape immune-surveillance. We designed EpCAM (epithelial cell adhesion molecule)-targeted cationic liposome (LPP-P4-Ep) containing si-CD47 and si-PD-L1 could target high-EpCAM cancer cells and knockdown both CD47 and PD-L1 proteins.
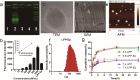
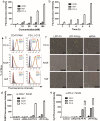
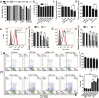
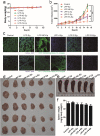
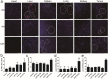
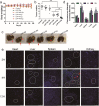
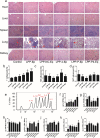
Findings: Efficient silencing of CD47 and PD-L1 versus single gene silencing in vivo by systemic administration of LPP-P4-Ep could significantly inhibited the growth of solid tumors in subcutaneous and reduced lung metastasis in lung metastasis model. Target delivery of the complexes LPP-P4-Ep increased anti-tumor T cell and NK cell response, and release various cytokines including IFN-γ and IL-6 in vivo and in vitro.
Interpretation: This multi-nanoparticles showed significantly high-EpCAM tumor targeting and lower toxicity, and enhanced immune therapeutic efficacy. Our data indicated that dual-blockade tumor cell-specific innate and adaptive checkpoints represents an improved strategy for tumor immunotherapy. FUND: This research supported by the Ministry of Science and Technology of the People's Republic of China (grant number 2015CB931804); the National Natural Science Foundation of China (NSFC, grant numbers 81703555, U1505225 and 81773063), and the China Postdoctoral Science Foundation (grant number 2017 M620268).
Keywords: CD47/SIPR-α; EpCAM targeted; Gene therapy; Immune therapy; Liposome; PD-L1/PD-1; siRNA.
Copyright © 2019. Published by Elsevier B.V.
Figures

References
-
- Dong H. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

