Role of agonist efficacy in exposure-induced enhancement of mu opioid reward in rats
- PMID: 30880123
- PMCID: PMC6500752
- DOI: 10.1016/j.neuropharm.2019.03.020
Role of agonist efficacy in exposure-induced enhancement of mu opioid reward in rats
Abstract
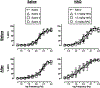
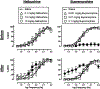
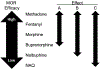
The abuse potential of opioid analgesics in humans appears to increase rapidly during initial regimens of opioid exposure. Previous work using intracranial self-stimulation (ICSS), a preclinical procedure useful for studying rewarding drug effects in drug-naïve animals, has similarly shown that rewarding effects of mu opioid receptor (MOR) agonists increase rapidly in rats during initial regimens of opioid administration. The goal of the present study was to evaluate the role of MOR agonist efficacy as a determinant in eliciting this trajectory of increased rewarding effects during initial opioid exposure in opioid-naïve rats. Separate groups of adult, male Sprague-Dawley rats responded for electrical brain stimulation using a frequency-rate ICSS procedure and received repeated daily treatment with vehicle or one of five MOR agonists that ranged from low to high efficacy (NAQ, nalbuphine, buprenorphine, fentanyl, methadone). Two additional groups were used to evaluate effects of repeated treatment with non-opioids (the cannabinoid CP55940 or the monoamine releaser amphetamine). Morphine was tested after each repeated treatment. In opioid-naïve rats tested before repeated dosing, MOR agonists produced primarily dose- and efficacy-dependent decreases in ICSS. Following repeated treatment, all MOR agonists except NAQ produced tolerance to opioid-induced rate-decreasing effects and enhanced expression of ICSS facilitation (indicative of opioid reward) by both the repeatedly administered drug and morphine. Repeated treatment with CP55940 and amphetamine produced different effects. Collectively, these results provide evidence to suggest that enhanced expression of opioid reward after initial regimens of opioid exposure has a low requirement for MOR agonist efficacy and is pharmacologically selective.
Keywords: Efficacy; Intracranial self-stimulation; Morphine; Mu opioid receptor; Repeated dosing.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Temporal parameters of enhanced opioid reward after initial opioid exposure in rats.Psychopharmacology (Berl). 2021 Mar;238(3):725-734. doi: 10.1007/s00213-020-05725-3. Epub 2021 Jan 7. Psychopharmacology (Berl). 2021. PMID: 33410983 Free PMC article.
-
Effectiveness comparisons of G-protein biased and unbiased mu opioid receptor ligands in warm water tail-withdrawal and drug discrimination in male and female rats.Neuropharmacology. 2019 May 15;150:200-209. doi: 10.1016/j.neuropharm.2019.01.020. Epub 2019 Jan 18. Neuropharmacology. 2019. PMID: 30660628 Free PMC article.
-
Effects of the novel, selective and low-efficacy mu opioid receptor ligand NAQ on intracranial self-stimulation in rats.Psychopharmacology (Berl). 2015 Feb;232(4):815-24. doi: 10.1007/s00213-014-3719-7. Epub 2014 Sep 3. Psychopharmacology (Berl). 2015. PMID: 25178814 Free PMC article.
-
Determinants of opioid abuse potential: Insights using intracranial self-stimulation.Peptides. 2019 Feb;112:23-31. doi: 10.1016/j.peptides.2018.10.007. Epub 2018 Nov 1. Peptides. 2019. PMID: 30391425 Free PMC article. Review.
-
Interactions between pain states and opioid reward assessed with intracranial self-stimulation in rats.Neuropharmacology. 2019 Dec 1;160:107689. doi: 10.1016/j.neuropharm.2019.107689. Epub 2019 Jul 1. Neuropharmacology. 2019. PMID: 31271771 Free PMC article. Review.
Cited by
-
Confronting the opioid crisis with basic research in neuropharmacology.Neuropharmacology. 2020 Apr;166:107972. doi: 10.1016/j.neuropharm.2020.107972. Epub 2020 Jan 17. Neuropharmacology. 2020. PMID: 31958407 Free PMC article. No abstract available.
-
Antinociceptive and Abuse Potential Effects of Cannabinoid/Opioid Combinations in a Chronic Pain Model in Rats.Brain Sci. 2019 Nov 17;9(11):328. doi: 10.3390/brainsci9110328. Brain Sci. 2019. PMID: 31744226 Free PMC article.
-
Changes in fentanyl demand following naltrexone, morphine, and buprenorphine in male rats.Drug Alcohol Depend. 2020 Feb 1;207:107804. doi: 10.1016/j.drugalcdep.2019.107804. Epub 2019 Dec 16. Drug Alcohol Depend. 2020. PMID: 31862556 Free PMC article.
-
Activation of mesocorticolimbic dopamine projections initiates cue-induced reinstatement of reward seeking in mice.Acta Pharmacol Sin. 2022 Sep;43(9):2276-2288. doi: 10.1038/s41401-022-00866-x. Epub 2022 Feb 25. Acta Pharmacol Sin. 2022. PMID: 35217811 Free PMC article.
-
Lack of effect of the nociceptin opioid peptide agonist Ro 64-6198 on pain-depressed behavior and heroin choice in rats.Drug Alcohol Depend. 2022 Feb 1;231:109255. doi: 10.1016/j.drugalcdep.2021.109255. Epub 2021 Dec 30. Drug Alcohol Depend. 2022. PMID: 34998256 Free PMC article.
References
-
- Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS, 2017. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol 31, 730–739. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

