Evaluating the Impact of Human Factors and Pen Needle Design on Insulin Pen Injection
- PMID: 30880448
- PMCID: PMC6501541
- DOI: 10.1177/1932296819836987
Evaluating the Impact of Human Factors and Pen Needle Design on Insulin Pen Injection
Abstract
Background: Limited published data exists quantifying the influence of human factors (HF) and pen needle (PN) design on delivery outcomes of pen injection systems. This preclinical in vivo study examines the impact of PN hub design and applied force against the skin during injection on needle penetration depth (NPD).
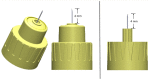
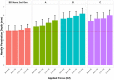
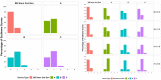
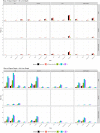
Method: To precisely locate injection depth, PN injections (20 µl; 2 IU, U-100 volume equivalent) of iodinated contrast agent were administered to the flank of Yorkshire swine across a range of clinically relevant application forces against the skin (0.25, 0.75, 1.25, and 2.0 lbf). The NPD, representing in vivo needle tip depth in SC tissue, from four 32 G × 4 mm PN devices (BD Nano™ 2nd Gen and three commercial posted-hub PN devices; n = 75/device/force, 1200 total) was measured by fluoroscopic imaging of the resulting depot.
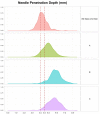
Results: The reengineered hub design more closely achieved the 4 mm target NPD with significantly less variability ( P = .006) than commercial posted-hub PN devices across the range of applied injection forces. Calculations of IM (intramuscular) injection risk completed through in silico probability model, using NPD and average human tissue thickness measurements, displayed a commensurate reduction (~2-8x) compared to conventional PN hub designs.
Conclusions: Quantifiable differences in injection depth were observed between identical labeled length PN devices indicating that hub design features, coupled with aspects of variable injection technique, may influence injection depth accuracy and consistency. The reengineered hub design may reduce the impact of unintended individual technique differences by improving target injection depth consistency and reducing IM injection potential.
Keywords: in vivo; injection depth; injection force variability; injection technique; intramuscular risk; pen needle.
Conflict of interest statement
Figures




References
-
- Laurent A, Mistretta F, Bottigioli D, et al. Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine. 2007;21;25:6423-6430. - PubMed
-
- Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin. 2010;26:1519-1530. - PubMed
-
- Ploin D, Schwarzenbach F, Dubray C, et al. Echographic measurement of skin thickness in sites suitable for intradermal vaccine injection in infants and children. Vaccine. 2011;29:8438-8442. - PubMed
-
- Ludescher B, Rommel M, Willmer T, Fritsche A, Schick F, Machann J. Subcutaneous adipose tissue thickness in adults—correlation with BMI and recommendations for pen needle lengths for subcutaneous self-injection. Clin Endocrinol (Oxf). 2011;75:786-790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

