Single-use suvorexant for treating insomnia during overnight polysomnography in patients with suspected obstructive sleep apnea: a single-center experience
- PMID: 30880914
- PMCID: PMC6400124
- DOI: 10.2147/DDDT.S197237
Single-use suvorexant for treating insomnia during overnight polysomnography in patients with suspected obstructive sleep apnea: a single-center experience
Abstract
Purpose: Although patients with suspected obstructive sleep apnea (OSA) might suffer difficulty in falling asleep during overnight polysomnography (PSG), standard hypnotics to obtain sleep during PSG have not been established. The aim of this study was to investigate the safety and efficacy of a new hypnotic agent, suvorexant, a dual orexin receptor antagonist, for insomnia in suspected OSA patients during in-laboratory PSG.
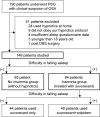
Patients and methods: An observational study was conducted during PSG for 149 patients with suspected OSA who had no insomnia at home. Patients with difficulty in falling asleep during PSG were optionally permitted to take single-use suvorexant. Patients with residual severe insomnia (>1 hour) after taking suvorexant were permitted to take an add-on use zolpidem. Clinical data and sleep questionnaire results were analyzed between a no insomnia group (without hypnotics) and an insomnia group (treated with suvorexant).
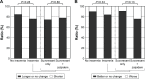
Results: Among 84 patients who experienced insomnia during PSG and required hypnotics (the insomnia group; treated with suvorexant), 44 (52.4%) achieved sufficient subjective sleep with single-use of suvorexant, while the other 40 (47.6%) required suvorexant plus zolpidem. An apnea hypopnea index (AHI) of ≥5 was observed in 144 out of 149 patients with predominantly obstructive respiratory events. Among those patients, 70.8% in the no insomnia group and 63.1% in the insomnia group had severe OSA. Regarding both subjective sleep time and morning mood, significant differences between the no insomnia group and the insomnia group were not observed. No patient taking suvorexant had an adverse event, such as delirium or falling.
Conclusion: Single-use suvorexant seems to be a safe and effective (but mild) hypnotic agent for suspected OSA patients with insomnia during in-laboratory PSG.
Keywords: insomnia; natural sleep; obstructive sleep apnea; polysomnography; suvorexant; zolpidem.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia.Drugs Today (Barc). 2016 Jan;52(1):29-40. doi: 10.1358/dot.2016.52.1.2439940. Drugs Today (Barc). 2016. PMID: 26937493 Review.
-
Suvorexant for insomnia in patients with psychiatric disorder: A 1-week, open-label study.Neuropsychopharmacol Rep. 2019 Sep;39(3):252-255. doi: 10.1002/npr2.12069. Epub 2019 Jul 8. Neuropsychopharmacol Rep. 2019. PMID: 31283862 Free PMC article. Clinical Trial.
-
Effects of Suvorexant, an Orexin Receptor Antagonist, on Respiration during Sleep In Patients with Obstructive Sleep Apnea.J Clin Sleep Med. 2016 Jan;12(1):9-17. doi: 10.5664/jcsm.5382. J Clin Sleep Med. 2016. PMID: 26194728 Free PMC article. Clinical Trial.
-
Clinical profile of suvorexant for the treatment of insomnia over 3 months in women and men: subgroup analysis of pooled phase-3 data.Psychopharmacology (Berl). 2017 Jun;234(11):1703-1711. doi: 10.1007/s00213-017-4573-1. Epub 2017 Mar 7. Psychopharmacology (Berl). 2017. PMID: 28265715 Clinical Trial.
-
Suvorexant in the Treatment of Difficulty Falling and Staying Asleep (Insomnia).Psychopharmacol Bull. 2022 Feb 25;52(1):68-90. Psychopharmacol Bull. 2022. PMID: 35342199 Free PMC article. Review.
Cited by
-
Suvorexant, a Novel Dual Orexin Receptor Antagonist, for the Management of Insomnia.Health Psychol Res. 2023 Jan 28;10(5):67898. doi: 10.52965/001c.67898. eCollection 2022. Health Psychol Res. 2023. PMID: 36726477 Free PMC article.
-
Active metabolites and potential mechanisms of Notopterygium incisum against obstructive sleep apnea Syndrome (OSAS): network analysis and experimental assessment.Front Pharmacol. 2023 Aug 31;14:1185100. doi: 10.3389/fphar.2023.1185100. eCollection 2023. Front Pharmacol. 2023. PMID: 37719850 Free PMC article.
References
-
- Newell J, Mairesse O, Verbanck P, Neu D. Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability in polysomnographic recordings among different clinical population samples. Psychiatry Res. 2012;200(2–3):795–801. - PubMed
-
- Agnew HW, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2(3):263–266. - PubMed
-
- Blackwell T, Paudel M, Redline S, Ancoli-Israel S, Stone KL, Osteoporotic Fractures in Men (MrOS) Study Group A novel approach using actigraphy to quantify the level of disruption of sleep by in-home polysomnography: the MROS sleep study: sleep disruption by poly-somnography. Sleep Med. 2017;32:97–104. - PMC - PubMed
-
- Curcio G, Ferrara M, Piergianni A, Fratello F, de Gennaro L. Paradoxes of the first-night effect: a quantitative analysis of antero-posterior EEG topography. Clin Neurophysiol. 2004;115(5):1178–1188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

