Aged kidneys are refractory to autophagy activation in a rat model of renal ischemia-reperfusion injury
- PMID: 30880933
- PMCID: PMC6402441
- DOI: 10.2147/CIA.S197444
Aged kidneys are refractory to autophagy activation in a rat model of renal ischemia-reperfusion injury
Abstract
Background: Ischemia-reperfusion (I/R) injury is the most common cause of acute kidney injury (AKI). Numerous therapeutic approaches for I/R injury have been studied, including autophagy, particularly in animal models of renal I/R injury derived from young or adult animals. However, the precise role of autophagy in renal ischemia-reperfusion in the aged animal model remains unclear. The purpose of this study was to demonstrate whether autophagy has similar effects on renal I/R injury in young and aged rats.
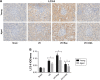
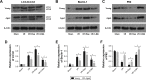
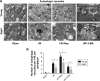
Materials and methods: All rats were divided into two age groups (3 months and 24 months) with each group being further divided into four subgroups (sham, I/R, I/R+Rap (rapamycin, an activator of autophagy), I/R+3-MA (3-methyladenine, an inhibitor of autophagy)). The I/R+Rap and I/R+3-MA groups were intraperitoneally injected with rapamycin and 3-MA prior to ischemia. We then measured serum levels of urea nitrogen, creatinine and assessed damage in the renal tissue. Immunohistochemistry was used to assess LC3-II and caspase-3, and Western blotting was used to evaluate the autophagy-related proteins LC3-II, Beclin-1 and P62. Apoptosis and autophagosomes were evaluated by TUNEL and transmission electron microscopy, respectively.
Results: Autophagy was activated in both young and aged rats by I/R and enhanced by rapamycin, although the level of autophagy was lower in the aged groups. In young rats, the activation of autophagy markedly improved renal function, reduced apoptosis in the renal tubular epithelial cells and the injury score in the renal tissue, thereby exerting protective effects on renal I/R injury. However, this level of protection was not present in aged rats.
Conclusion: Our data indicated that the activation of autophagy was ineffective in aged rat kidneys. These discoveries may have major implications in that severe apoptosis in aged kidneys might be refractory to antiapoptotic effect induced by the activation of autophagy.
Keywords: 3-methyladenine; aged; apoptosis; autophagy; ischemia-reperfusion; rapamycin; renal.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
-
- Mehta RL, Burdmann EA, Cerdá J, et al. Recognition and management of acute kidney injury in the International Society of nephrology 0 by 25 global snapshot: a multinational cross-sectional study. Lancet. 2016;387(10032):2017–2025. - PubMed
-
- Kunzendorf U, Haase M, Rölver L, Haase-Fielitz A. Novel aspects of pharmacological therapies for acute renal failure. Drugs. 2010;70(9):1099–1114. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

