Efficacy of aclidinium/formoterol 400/12 µg, analyzed by airflow obstruction severity, age, sex, and exacerbation history: pooled analysis of ACLIFORM and AUGMENT
- PMID: 30880938
- PMCID: PMC6396834
- DOI: 10.2147/COPD.S185502
Efficacy of aclidinium/formoterol 400/12 µg, analyzed by airflow obstruction severity, age, sex, and exacerbation history: pooled analysis of ACLIFORM and AUGMENT
Abstract
Background: Aclidinium/formoterol 400/12 µg is a twice-daily maintenance bronchodilator for COPD. This post hoc study evaluated aclidinium/formoterol vs aclidinium 400 µg, formoterol 12 µg, or placebo in patient subgroups.
Patients and methods: Data were pooled from two 24-week Phase III clinical trials (ACLIFORM and AUGMENT). Patients (N=3,394) were analyzed by baseline airflow obstruction severity (moderate/severe), age (<65/≥65 years), sex, and exacerbation history (0/≥1 exacerbation in the previous 12 months). Changes from baseline vs placebo and mono-therapies were evaluated: morning pre-dose (trough) and morning 1-hour post-dose FEV1, Transition Dyspnea Index (TDI), and moderate/severe exacerbation rates (healthcare resource utilization [HCRU] and EXAcerbations of Chronic pulmonary disease Tool [EXACT] criteria).
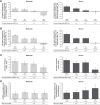
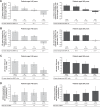
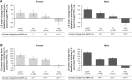
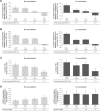
Results: Aclidinium/formoterol improved the post-dose FEV1 vs placebo and monotherapy in all subgroups (all P<0.01) and trough FEV1 vs placebo (P<0.001) and formoterol (P<0.05) across all subgroups. Improvements in trough FEV1 were observed vs aclidinium in patients with severe airflow obstruction, patients aged <65 years, males, and patients with exacerbation history (P<0.05). Improvements in TDI were observed vs placebo in all subgroups (all P<0.001), monotherapies for patients with moderate (formoterol P<0.05) or severe airflow obstruction (aclidinium P<0.05), patients aged <65 years (aclidinium P<0.01, formoterol P<0.05), males (formoterol P<0.05), and patients with no exacerbation history (formoterol P<0.05). HCRU exacerbation rates were lower for aclidinium/formoterol vs placebo in patients with no exacerbation history (P<0.01). EXACT exacerbation rates were lower for aclidinium/formoterol in patients with moderate airflow obstruction vs placebo and aclidinium, patients aged <65 years vs placebo and ≥65 years vs formoterol, males vs placebo, and patients with no exacerbation history vs placebo (all P<0.05).
Conclusion: Aclidinium/formoterol significantly improved post-dose FEV1, trough FEV1, and TDI vs placebo across all subgroups and vs monotherapy in many subgroups. These findings further support the benefits of aclidinium/formoterol for all patients with COPD.
Keywords: COPD; aclidinium; formoterol.
Conflict of interest statement
Disclosure ADD has received research, consulting, and lecturing fees from Almirall S.A., Altana, AstraZeneca (AZ), Boehringer Ingelheim (BI; Canada) Ltd., Forest Laboratories LLC, GlaxoSmithKline (GSK), KOS Pharmaceuticals, Merck Canada, Methapharm, Novartis ([Nov]; Canada/USA), ONO Pharmaceutical, Pfizer Canada, Schering-Plough, Sepracor Inc., and SkyePharma. DS has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards, and research grants from various pharmaceutical companies including Apellis Pharmaceuticals, AZ, BI, Chiesi Farmaceutici S.p.A, Cipla, Genentech, Glenmark Pharmaceuticals, GSK, Johnson and Johnson, Mundipharma, Nov, Peptinnovate Ltd., Pfizer Inc., Pulmatrix, Skyepharma, Teva Pharmaceutical Industries Ltd., Theravance Biopharma, and Verona Pharma. JFD has received consulting fees from AZ, BI, Circassia, GSK, and Sunovion. He is a member of the Data Monitoring Committee for AZ. EMK has participated in consulting, advisory boards, speaker panels, or received travel reimbursement from Amphastar Pharmaceuticals, AZ, Forest Laboratories LLC, GSK, Mylan, Nov, Oriel, Pearl Therapeutics, Sunovion, Teva Pharmaceutical Industries Ltd., and Theravance Biopharma. He has conducted multicenter clinical research trials for ~40 pharmaceutical companies. AR, EM, FC, DJ, and EGG are employees of AZ and former employees of Almirall S.A., Barcelona, Spain. The authors report no other conflicts of interest in this work.
Figures




References
-
- Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2018. [Accessed October 11, 2018]. Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-rev....
-
- Jenkins CR, Chapman KR, Donohue JF, Roche N, Tsiligianni I, Han MK. Improving the management of COPD in women. Chest. 2017;151(3):686–696. - PubMed
-
- Singh D, Roche N, Halpin D, Agusti A, Wedzicha JA, Martinez FJ. Current controversies in the pharmacological treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194(5):541–549. - PubMed
-
- Godtfredsen NS, Lam TH, Hansel TT, et al. COPD-related morbidity and mortality after smoking cessation: status of the evidence. Eur Respir J. 2008;32(4):844–853. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

