Extrafine triple therapy delays COPD clinically important deterioration vs ICS/LABA, LAMA, or LABA/LAMA
- PMID: 30880943
- PMCID: PMC6400232
- DOI: 10.2147/COPD.S196383
Extrafine triple therapy delays COPD clinically important deterioration vs ICS/LABA, LAMA, or LABA/LAMA
Abstract
Background: Current pharmacological therapies for COPD improve quality of life and symptoms and reduce exacerbations. Given the progressive nature of COPD, it is arguably more important to understand whether the available therapies are able to delay clinical deterioration; the concept of "clinically important deterioration" (CID) has therefore been developed. We evaluated the efficacy of the single-inhaler triple combination beclometasone dipropionate, formoterol fumarate, and glycopyrronium (BDP/FF/G), using data from three large 1-year studies.
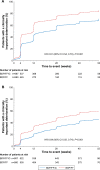
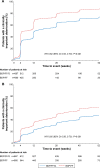
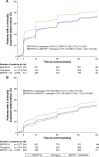
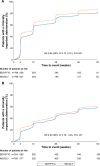
Methods: The studies compared BDP/FF/G to BDP/FF (TRILOGY), tiotropium (TRINITY), and indacaterol/glycopyrronium (IND/GLY; TRIBUTE). All studies recruited patients with symptomatic COPD, FEV1 <50%, and an exacerbation history. We measured the time to first CID and to sustained CID, an endpoint combining FEV1, St George's Respiratory Questionnaire (SGRQ), moderate-to-severe exacerbations, and death. The time to first CID was based on the first occurrence of any of the following: a decrease of ≥100 mL from baseline in FEV1, an increase of ≥4 units from baseline in SGRQ total score, the occurrence of a moderate/severe COPD exacerbation, or death. The time to sustained CID was defined as: a CID in FEV1 and/or SGRQ total score maintained at all subsequent visits, an exacerbation, or death.
Results: Extrafine BDP/FF/G significantly extended the time to first CID vs BDP/FF (HR 0.61, P<0.001), tiotropium (0.72, P<0.001), and IND/GLY (0.82, P<0.001), and significantly extended the time to sustained CID vs BDP/FF (HR 0.64, P<0.001) and tiotropium (0.80, P<0.001), with a numerical extension vs IND/GLY.
Conclusion: In patients with symptomatic COPD, FEV1 <50%, and an exacerbation history, extrafine BDP/FF/G delayed disease deterioration compared with BDP/FF, tiotropium, and IND/GLY.
Trial registration: The studies are registered in ClinicalTrials.gov: TRILOGY, NCT01917331; TRINITY, NCT01911364; TRIBUTE, NCT02579850.
Keywords: anticholinergics; beta-2 agonists; chronic obstructive pulmonary disease; disease activity; inhaled corticosteroids.
Conflict of interest statement
Disclosure DS is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC). DS received personal fees from Chiesi during the conduct of these studies. Outside the submitted work, DS reports grants and personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Glenmark, Johnson and Johnson, Merck, NAPP, Novartis, Pfizer, Takeda, Teva, Theravance, and Verona, and personal fees from Genentech and Skyepharma. LMF reports grants, personal fees, and non-financial support from Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Merck Sharp & Dohme, Takeda, AstraZeneca, Novartis, Menarini, Laboratori Guidotti, and Almirall; personal fees and non-financial support from Pearl Therapeutics, Mundipharma, and Boston Scientific; personal fees from Kyorin, Bayer, and Zambon; and grants from Pfizer, Dompè, Malesci, Alfasigma, and Vree Health Italia, all outside the submitted work. SV and SP are employed by Chiesi, the sponsor of the studies. AP reports grants, personal fees, non-financial support, and advisory board membership from Chiesi, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Mundipharma, and TEVA; personal fees and non-financial support from Menarini, Novartis, and Zambon; and grants from Sanofi, all outside the submitted work. The authors report no other conflicts of interest in this work.
Figures
References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

