Ultrafine particles in airways: a novel marker of COPD exacerbation risk and inflammatory status
- PMID: 30880945
- PMCID: PMC6402613
- DOI: 10.2147/COPD.S187560
Ultrafine particles in airways: a novel marker of COPD exacerbation risk and inflammatory status
Erratum in
-
Ultrafine particles in airways: a novel marker of COPD exacerbation risk and inflammatory status [Corrigendum].Int J Chron Obstruct Pulmon Dis. 2019 Mar 27;14:739-740. doi: 10.2147/COPD.S208588. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31114179 Free PMC article. No abstract available.
Abstract
Purpose: Ultrafine particles (UFP) are toxic due to their small size and penetration into deeper lung compartments. We aimed to evaluate the exhaled breath condensate (EBC)-UFP content as a reflection of inflammation and oxidative stress status in COPD patients and as an exacerbation risk marker.
Methods: EBC was collected by conventional methods. Particles were analyzed with NanoSight LM20. EBC carbonyl and 8-hydroxydeoxyguanosine (8-OHdG) levels were measured using ELISA kits. Study population (58 COPD patients and 40 healthy smoker and non-smoker controls) underwent spirometry, diffusion capacity, EBC testing, and blood sampling.
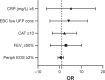
Results: Absolute eosinophil count, C-reactive protein (CRP), and lactate dehydrogenase in serum were elevated in the COPD group compared with the controls (224 U/L, 5 mg/L, and 391 U/L vs 154 U/L, 3 mg/L, and 330 U/L, P=0.009, P=0.05, and P=0.004, respectively). COPD patients had lower UFP concentrations in EBC compared with controls (0.24 E8/mL vs 0.51 E8/mL, P≤0.001). A mirror image was detected in serum: COPD patients had higher UFP concentrations compared with controls (9.8 E8/mL vs 6.7 E8/mL, respectively, P=0.03). EBC carbonyl and 8-OHdG levels were higher among COPD patients compared with controls (5.1 per 1 µg/mL protein and 0.036 ng/mL vs 0.41 per 1 µg/mL protein and 0.003 ng/mL, P=0.001 and P≤0.001, respectively). EBC UFP concentrations were negatively correlated with pack years (R=-0.44, P ≤0.001) and positively correlated with FEV1 and diffusing lung capacity for carbon monoxide (R=0.46, 0.23, P ≤0.001 and P=0.04, respectively). Low EBC UFP concentrations (≤0.18 E8/mL) and CRP levels ≥5 mg/L were independent predictors of the frequent exacerbator phenotype (OR 3.6; 95% CI: 1.06-7.97; P=0.04 and OR 4.4; 95% CI: 1.24-10.2; P=0.02, respectively).
Conclusion: UFP content in EBC reflects the inflammatory state of airways. Low UFP concentrations in EBC and high in serum of COPD patients support our hypothesis that increased epithelial permeability could be the mechanism behind those findings.
Keywords: COPD; EBC; exhaled breath condensate; oxidative stress.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Inflammatory mediators in exhaled breath condensate and peripheral blood of healthy donors and stable COPD patients.Immunopharmacol Immunotoxicol. 2019 Apr;41(2):224-230. doi: 10.1080/08923973.2019.1609496. Epub 2019 May 3. Immunopharmacol Immunotoxicol. 2019. PMID: 31046512 Clinical Trial.
-
Exhaled breath condensate pH as a biomarker of COPD severity in ex-smokers.Respir Res. 2011 May 22;12(1):67. doi: 10.1186/1465-9921-12-67. Respir Res. 2011. PMID: 21600044 Free PMC article.
-
Cigarette-related cadmium and environmental pollution exposure are reflected in airway ultrafine particle content.ERJ Open Res. 2020 Sep 14;6(3):00361-2019. doi: 10.1183/23120541.00361-2019. eCollection 2020 Jul. ERJ Open Res. 2020. PMID: 32963996 Free PMC article.
-
Oxidative stress in COPD and its measurement through exhaled breath condensate.Clin Transl Sci. 2009 Apr;2(2):150-5. doi: 10.1111/j.1752-8062.2009.00093.x. Clin Transl Sci. 2009. PMID: 20443881 Free PMC article. Review.
-
Exhaled biomarkers.Chest. 2006 Nov;130(5):1541-6. doi: 10.1378/chest.130.5.1541. Chest. 2006. PMID: 17099035 Review.
Cited by
-
Impact of smoking on redox homeostasis and 8-OHdG levels in COPD patients: a cross-sectional comparative study.BMC Pulm Med. 2025 Sep 2;25(1):423. doi: 10.1186/s12890-025-03907-3. BMC Pulm Med. 2025. PMID: 40898117 Free PMC article.
-
COVID-19 Lockdown in Israel: The Environmental Effect on Ultrafine Particle Content in the Airway.Int J Environ Res Public Health. 2022 May 1;19(9):5507. doi: 10.3390/ijerph19095507. Int J Environ Res Public Health. 2022. PMID: 35564902 Free PMC article.
-
Trajectory of inhaled cadmium ultrafine particles in smokers.BMJ Open Respir Res. 2021 Nov;8(1):e001000. doi: 10.1136/bmjresp-2021-001000. BMJ Open Respir Res. 2021. PMID: 34845007 Free PMC article.
-
Implications of DNA damage in chronic lung disease.Front Cell Dev Biol. 2024 Oct 31;12:1436767. doi: 10.3389/fcell.2024.1436767. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39544366 Free PMC article. Review.
-
Biomarkers of the relationship of particulate matter exposure with the progression of chronic respiratory diseases.Korean J Intern Med. 2024 Jan;39(1):25-33. doi: 10.3904/kjim.2023.393. Epub 2024 Jan 1. Korean J Intern Med. 2024. PMID: 38225823 Free PMC article.
References
-
- Li N, Georas S, Alexis N, et al. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J Allergy Clin Immunol. 2016;138(2):386–396. - PMC - PubMed
-
- Bar-Shai A, Alcalay Y, Sagiv A, et al. Fingerprint of lung fluid ultrafine particles, a novel marker of acute lung inflammation. Respiration. 2015;90(1):74–84. - PubMed
-
- Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–122. - PubMed
-
- Gan WQ, Fitzgerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187(7):721–727. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

