Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa
- PMID: 30880959
- PMCID: PMC6396885
- DOI: 10.2147/IJN.S191340
Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa
Abstract
Background: The threat of drug-resistant Pseudomonas aeruginosa requires great efforts to develop highly effective and safe bactericide.
Objective: This study aimed to investigate the antibacterial activity and mechanism of silver nanoparticles (AgNPs) against multidrug-resistant P. aeruginosa.
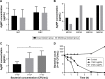
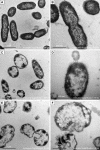
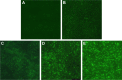
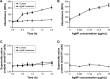
Methods: The antimicrobial effect of AgNPs on clinical isolates of resistant P. aeruginosa was assessed by minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC). In multidrug-resistant P. aeruginosa, the alterations of morphology and structure were observed by the transmission electron microscopy (TEM); the differentially expressed proteins were analyzed by quantitative proteomics; the production of reactive oxygen species (ROS) was assayed by H2DCF-DA staining; the activity of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) was chemically measured and the apoptosis-like effect was determined by flow cytometry.
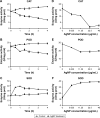
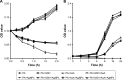
Results: Antimicrobial tests revealed that AgNPs had highly bactericidal effect on the drug-resistant or multidrug-resistant P. aeruginosa with the MIC range of 1.406-5.625 µg/mL and the MBC range of 2.813-5.625 µg/mL. TEM showed that AgNPs could enter the multidrug-resistant bacteria and impair their morphology and structure. The proteomics quantified that, in the AgNP-treated bacteria, the levels of SOD, CAT, and POD, such as alkyl hydroperoxide reductase and organic hydroperoxide resistance protein, were obviously high, as well as the significant upregulation of low oxygen regulatory oxidases, including cbb3-type cytochrome c oxidase subunit P2, N2, and O2. Further results confirmed the excessive production of ROS. The antioxidants, reduced glutathione and ascorbic acid, partially antagonized the antibacterial action of AgNPs. The apoptosis-like rate of AgNP-treated bacteria was remarkably higher than that of the untreated bacteria (P<0.01).
Conclusion: This study proved that AgNPs could play antimicrobial roles on the multidrug-resistant P. aeruginosa in a concentration- and time-dependent manner. The main mechanism involves the disequilibrium of oxidation and antioxidation processes and the failure to eliminate the excessive ROS.
Keywords: AgNPs; Pseudomonas aeruginosa; antibacterial activity; mechanism; multidrug-resistant bacterium; silver nanoparticles.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Silver nanoparticle with potential antimicrobial and antibiofilm efficiency against multiple drug resistant, extensive drug resistant Pseudomonas aeruginosa clinical isolates.BMC Microbiol. 2024 Jul 26;24(1):277. doi: 10.1186/s12866-024-03397-z. BMC Microbiol. 2024. PMID: 39060955 Free PMC article.
-
Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy.Int J Mol Sci. 2017 Mar 6;18(3):569. doi: 10.3390/ijms18030569. Int J Mol Sci. 2017. PMID: 28272303 Free PMC article.
-
In vitro and in vivo antimicrobial activity of combined therapy of silver nanoparticles and visible blue light against Pseudomonas aeruginosa.Int J Nanomedicine. 2016 Apr 27;11:1749-58. doi: 10.2147/IJN.S102398. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27175075 Free PMC article.
-
Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains.Braz J Microbiol. 2021 Mar;52(1):267-278. doi: 10.1007/s42770-020-00406-x. Epub 2020 Nov 24. Braz J Microbiol. 2021. PMID: 33231865 Free PMC article. Review.
-
Silver Nanoparticles and Their Antibacterial Applications.Int J Mol Sci. 2021 Jul 4;22(13):7202. doi: 10.3390/ijms22137202. Int J Mol Sci. 2021. PMID: 34281254 Free PMC article. Review.
Cited by
-
Silver Nanoparticles on Chitosan/Silica Nanofibers: Characterization and Antibacterial Activity.Int J Mol Sci. 2019 Dec 25;21(1):166. doi: 10.3390/ijms21010166. Int J Mol Sci. 2019. PMID: 31881739 Free PMC article.
-
Silver Is Not Equal to Silver: Synthesis and Evaluation of Silver Nanoparticles with Low Biological Activity, and Their Incorporation into C12Alanine-Based Hydrogel.Molecules. 2023 Jan 25;28(3):1194. doi: 10.3390/molecules28031194. Molecules. 2023. PMID: 36770861 Free PMC article.
-
Gallium-Curcumin Nanoparticle Conjugates as an Antibacterial Agent against Pseudomonas aeruginosa: Synthesis and Characterization.ACS Omega. 2022 Feb 17;7(8):6795-6809. doi: 10.1021/acsomega.1c06398. eCollection 2022 Mar 1. ACS Omega. 2022. PMID: 35252674 Free PMC article.
-
Cytotoxicity and Antibacterial Efficacy of AgCu and AgFe NanoAlloys: A Comparative Study.Antibiotics (Basel). 2022 Dec 1;11(12):1737. doi: 10.3390/antibiotics11121737. Antibiotics (Basel). 2022. PMID: 36551394 Free PMC article.
-
Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles.Microorganisms. 2022 Feb 14;10(2):437. doi: 10.3390/microorganisms10020437. Microorganisms. 2022. PMID: 35208891 Free PMC article. Review.
References
-
- Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2(9):1051–1060. - PubMed
-
- Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67(3):159–173. - PubMed
-
- Bjarnsholt T, Tolker-Nielsen T, Høiby N, Givskov M. Interference of Pseudomonas aeruginosa signalling and biofilm formation for infection control. Expert Rev Mol Med. 2010;12:e11. - PubMed
-
- Chen J, Chen Y, Hu P, Zhou T, Xu X, Pei X. Risk assessment of infected children with Pseudomonas aeruginosa pneumonia by combining host and pathogen predictors. Infect Genet Evol. 2018;57:82–87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

