Preparation of sustained release apremilast-loaded PLGA nanoparticles: in vitro characterization and in vivo pharmacokinetic study in rats
- PMID: 30880967
- PMCID: PMC6402442
- DOI: 10.2147/IJN.S195048
Preparation of sustained release apremilast-loaded PLGA nanoparticles: in vitro characterization and in vivo pharmacokinetic study in rats
Abstract
Background: Apremilast (APM) is a novel, orally administered small molecule drug approved for treatment of psoriasis or psoriatic arthritis. Due to its low solubility and permeability, it is classified as a class IV drug according to BCS classification. Dose titration is recommended during APM treatment due to its tolerability and twice-daily dosing regimen issues.
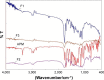
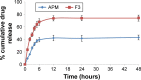
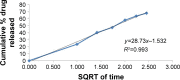
Materials and methods: In this study, three different APM-loaded PLGA nanoparticles (F1-F3) were prepared by single emulsion and evaporation method. Based on particle size, PDI, zeta potential (ZP), entrapment efficiency (%EE), drug loading (%DL), and spectral characterization, the nanoparticles (F3) were optimized. The F3 nanoparticles were further evaluated for in vitro release and in vivo pharmacokinetic studies in rats.
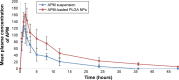
Results: The optimized nanoparticles (F3) had particles size 307.3±8.5 nm with a low PDI value 0.317, ZP of -43.4±2.6 mV, EE of 61.1±1.9% and DL of 1.9±0.1%. The in vitro release profile showed a sustained release pattern of F3 nanoparticles of APM. The pharmacokinetic results showed 2.25 times increase in bio-availability of F3 nanoparticles compared to normal APM suspension. Moreover, significant increase in half-life and mean residence time confirms long-term retention of F3 nanoparticles.
Conclusion: Bioavailability enhancement along-with long-term retention of the APM-loaded PLGA nanoparticles might be helpful for the once-daily regimen treatment.
Keywords: Poly(D,L-lactide-coglycolide); apremilast; bioavailability; nanoparticles; sustained release.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



Similar articles
-
In vitro-in vivo evaluation of chitosan-PLGA nanoparticles for potentiated gastric retention and anti-ulcer activity of diosmin.Int J Nanomedicine. 2019 Sep 4;14:7191-7213. doi: 10.2147/IJN.S213836. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31564873 Free PMC article.
-
Poly(lactic-co-glycolic acid)-loaded nanoparticles of betulinic acid for improved treatment of hepatic cancer: characterization, in vitro and in vivo evaluations.Int J Nanomedicine. 2018 Feb 16;13:975-990. doi: 10.2147/IJN.S157391. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29497292 Free PMC article.
-
Intensification of quercetin nanobubble formulation and performance by multi-factor optimization and interaction analysis.Pharm Dev Technol. 2025 Jan;30(1):10-24. doi: 10.1080/10837450.2024.2441182. Epub 2024 Dec 31. Pharm Dev Technol. 2025. PMID: 39718513
-
Brigatinib loaded poly(d,l-lactide-co-glycolide) nanoparticles for improved anti-tumoral activity against non-small cell lung cancer cell lines.Drug Dev Ind Pharm. 2021 Jul;47(7):1112-1120. doi: 10.1080/03639045.2021.1983585. Epub 2021 Oct 1. Drug Dev Ind Pharm. 2021. PMID: 34551665
-
Formulation optimization of etoposide loaded PLGA nanoparticles by double factorial design and their evaluation.Curr Drug Deliv. 2010 Jan;7(1):51-64. doi: 10.2174/156720110790396517. Curr Drug Deliv. 2010. PMID: 20044908 Review.
Cited by
-
Apremilast Microemulsion as Topical Therapy for Local Inflammation: Design, Characterization and Efficacy Evaluation.Pharmaceuticals (Basel). 2020 Dec 21;13(12):484. doi: 10.3390/ph13120484. Pharmaceuticals (Basel). 2020. PMID: 33371334 Free PMC article.
-
Investigating the Impact of Optimized Trans-Cinnamic Acid-Loaded PLGA Nanoparticles on Epithelial to Mesenchymal Transition in Breast Cancer.Int J Nanomedicine. 2022 Feb 18;17:733-750. doi: 10.2147/IJN.S345870. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35210772 Free PMC article.
-
Fabrication and characterization of apremilast-loaded zinc oxide-mesoporous silica nanoparticles for psoriasis treatment.Ther Deliv. 2024;15(6):449-462. doi: 10.1080/20415990.2024.2343646. Epub 2024 May 22. Ther Deliv. 2024. PMID: 38888579 Free PMC article.
-
Thymoquinone-entrapped chitosan-modified nanoparticles: formulation optimization to preclinical bioavailability assessments.Drug Deliv. 2021 Dec;28(1):973-984. doi: 10.1080/10717544.2021.1927245. Drug Deliv. 2021. PMID: 34036860 Free PMC article.
-
Biochemical, Ameliorative and Cytotoxic Effects of Newly Synthesized Curcumin Microemulsions: Evidence from In Vitro and In Vivo Studies.Nanomaterials (Basel). 2021 Mar 23;11(3):817. doi: 10.3390/nano11030817. Nanomaterials (Basel). 2021. PMID: 33806829 Free PMC article.
References
-
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850. - PubMed
-
- Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58(5):851–864. - PubMed
-
- Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75(12):1393–1403. - PubMed
-
- Hoffman MB, Hill D, Feldman SR. Current challenges and emerging drug delivery strategies for the treatment of psoriasis. Expert Opin Drug Deliv. 2016;13(10):1461–1473. - PubMed
-
- Assessment report of Apremilast (Otezla) Committee for medicinal products for human use. European Medicines Agency. 2014
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

