Preparation of sustained release apremilast-loaded PLGA nanoparticles: in vitro characterization and in vivo pharmacokinetic study in rats
- PMID: 30880967
- PMCID: PMC6402442
- DOI: 10.2147/IJN.S195048
Preparation of sustained release apremilast-loaded PLGA nanoparticles: in vitro characterization and in vivo pharmacokinetic study in rats
Abstract
Background: Apremilast (APM) is a novel, orally administered small molecule drug approved for treatment of psoriasis or psoriatic arthritis. Due to its low solubility and permeability, it is classified as a class IV drug according to BCS classification. Dose titration is recommended during APM treatment due to its tolerability and twice-daily dosing regimen issues.
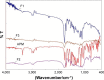
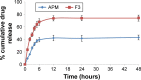
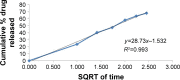
Materials and methods: In this study, three different APM-loaded PLGA nanoparticles (F1-F3) were prepared by single emulsion and evaporation method. Based on particle size, PDI, zeta potential (ZP), entrapment efficiency (%EE), drug loading (%DL), and spectral characterization, the nanoparticles (F3) were optimized. The F3 nanoparticles were further evaluated for in vitro release and in vivo pharmacokinetic studies in rats.
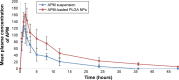
Results: The optimized nanoparticles (F3) had particles size 307.3±8.5 nm with a low PDI value 0.317, ZP of -43.4±2.6 mV, EE of 61.1±1.9% and DL of 1.9±0.1%. The in vitro release profile showed a sustained release pattern of F3 nanoparticles of APM. The pharmacokinetic results showed 2.25 times increase in bio-availability of F3 nanoparticles compared to normal APM suspension. Moreover, significant increase in half-life and mean residence time confirms long-term retention of F3 nanoparticles.
Conclusion: Bioavailability enhancement along-with long-term retention of the APM-loaded PLGA nanoparticles might be helpful for the once-daily regimen treatment.
Keywords: Poly(D,L-lactide-coglycolide); apremilast; bioavailability; nanoparticles; sustained release.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



References
-
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850. - PubMed
-
- Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58(5):851–864. - PubMed
-
- Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75(12):1393–1403. - PubMed
-
- Hoffman MB, Hill D, Feldman SR. Current challenges and emerging drug delivery strategies for the treatment of psoriasis. Expert Opin Drug Deliv. 2016;13(10):1461–1473. - PubMed
-
- Assessment report of Apremilast (Otezla) Committee for medicinal products for human use. European Medicines Agency. 2014
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

