Solid lipid nanoparticles with enteric coating for improving stability, palatability, and oral bioavailability of enrofloxacin
- PMID: 30880969
- PMCID: PMC6402439
- DOI: 10.2147/IJN.S183479
Solid lipid nanoparticles with enteric coating for improving stability, palatability, and oral bioavailability of enrofloxacin
Abstract
Background: The poor palatability, variable oral bioavailability, stimulation to gastric mucosa, and light instability limited the application of enrofloxacin (ENR). The enteric granules combining solid lipid nanoparticles (SLNs) with enteric coating were explored to overcome these disadvantages.
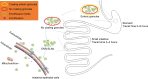
Materials and methods: ENR-loaded SLNs were produced by a hot homogenization and ultrasonic emulsification method and the enteric granules with SLNs as inner core were prepared by wet granulation followed by coating using polyacrylic resin II (PRII). The formulation was optimized by using orthogonal or single factor test screening.
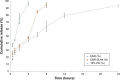
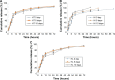
Results: The optimal SLNs with loading capacity (LC) and price as inspection indexes were consisted of 10 mL 3% polyvinyl alcohol per 0.8 g ENR and 2.4 g octadecanoic acid. The sizes, LC, polydispersion index, and zeta potential of the SLNs were 308.5±6.3 nm, 15.73%±0.31%, 0.352±0.015, and -22.3 mv, respectively. The best enteric granules were used 15% PRII as coating materials. The release of the enteric granules in simulated intestine fluid (SIF, pH=8) was significantly faster than in simulated gastric fluid (SGF, pH=2) and simultaneously slower than those of SLNs and native ENR. The granules showed good stability in influencing factor experiment. The granules displayed a similar daily feed intake as the control group and higher daily feed intake than ENR powder and single-coating granules. Compared to the ENR soluble powder, the area under the plasma concentration-time curve and mean retention time of the enteric granules after intragastric administration were increased from 4.26±0.85 µg h/mL and 6.80±2.28 hours to 11.24±3.33 µg h/mL and 17.97±4.01 hours, respectively.
Conclusion: The enteric granules combination SLNs with enteric coating significantly improved the stability, palatability, sustained-release performance and oral bioavailability of ENR. The novel technology will be a potential measure to overcome the similar disadvantages of other drugs.
Keywords: bioavailability; enrofloxacin; enteric coating; light stability; palatability; solid lipid nanoparticles.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




References
-
- Ribeiro C, Lopes SC, Gameiro P. New insights into the translocation route of enrofloxacin and its metalloantibiotics. J Membr Biol. 2011;241(3):117–125. - PubMed
-
- Liu M, Yin D, Fu H, et al. Double-coated enrofloxacin microparticles with chitosan and alginate: preparation, characterization and taste-masking effect study. Carbohydr Polym. 2017;170:247–253. - PubMed
-
- Chun MK, Choi HK. Preparation and characterization of enrofloxacin/carbopol complex in aqueous solution. Arch Pharm Res. 2004;27(6):670–675. - PubMed
-
- Zj L, Dong JN, Zeng BG. The new research progress of animals’ physiological taste and application of sweetening agent. Feed Industry. 2005;26(20):1–4.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

