A novel strategy of transferring NIS protein to cells using extracellular vesicles leads to increase in iodine uptake and cytotoxicity
- PMID: 30880979
- PMCID: PMC6413815
- DOI: 10.2147/IJN.S189738
A novel strategy of transferring NIS protein to cells using extracellular vesicles leads to increase in iodine uptake and cytotoxicity
Abstract
Background: This study was designed to explore a novel approach for transferring NIS protein to cells using extracellular vesicle (EV) and enhancing iodine avidity in hepatocellular carcinoma (HCC) cells.
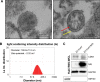
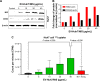
Methods: We transfected the HCC cells (Huh7) with NIS gene, designated as Huh7/NIS, and isolated the EVs from them. Presence of NIS protein in EVs and EV-mediated transport of NIS protein to recipient Huh7 cells were tested using Western blotting. We also examined radioiodine uptake in Huh7 cells treated with EV-Huh7/NIS.
Results: Successful transfer of NIS protein into Huh7 cells was confirmed by WB and microscopy. EVs showed high levels of NIS protein in them. Treatment of Huh7 cells with EV-Huh7/NIS increased the NIS protein level and enhanced 125I uptake in recipient Huh7 cells. In addition, EV-huh7/NIS pre-treatment enhanced the cytotoxicity of 131I therapy against Huh7 cells by inducing increased DNA damage/increased γH2A.X foci formation.
Conclusion: This is the first-of-its-kind demonstration of successful transportation of the NIS protein to cells via EVs, which increased radioiodine uptake. This approach can revert radioiodine-resistant cancers into radioiodine-sensitive cancers.
Keywords: extracellular vesicle; hepato-cellular carcinoma; iodine uptake; sodium iodide symporter (NIS).
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Extracellular vesicles deliver sodium iodide symporter protein and promote cancer cell radioiodine therapy.Sci Rep. 2022 Jul 1;12(1):11190. doi: 10.1038/s41598-022-15524-9. Sci Rep. 2022. PMID: 35778503 Free PMC article.
-
Radiation-Induced Amplification of TGFB1-Induced Mesenchymal Stem Cell-Mediated Sodium Iodide Symporter (NIS) Gene 131I Therapy.Clin Cancer Res. 2019 Oct 1;25(19):5997-6008. doi: 10.1158/1078-0432.CCR-18-4092. Epub 2019 Jun 13. Clin Cancer Res. 2019. PMID: 31196853
-
Systemic tumor-targeted sodium iodide symporter (NIS) gene therapy of hepatocellular carcinoma mediated by B6 peptide polyplexes.J Gene Med. 2017 May;19(5). doi: 10.1002/jgm.2957. J Gene Med. 2017. PMID: 28423213
-
The importance of sodium/iodide symporter (NIS) for thyroid cancer management.Arq Bras Endocrinol Metabol. 2007 Jul;51(5):672-82. doi: 10.1590/s0004-27302007000500004. Arq Bras Endocrinol Metabol. 2007. PMID: 17891230 Review.
-
Approaches to gene therapy with sodium/iodide symporter.Exp Clin Endocrinol Diabetes. 2001;109(1):56-9. doi: 10.1055/s-2001-11020. Exp Clin Endocrinol Diabetes. 2001. PMID: 11573142 Review.
Cited by
-
The effect of sodium iodide symporter protein on ablation success in patients with differentiated thyroid cancer.Ann Nucl Med. 2022 Dec;36(12):1050-1058. doi: 10.1007/s12149-022-01794-w. Epub 2022 Oct 10. Ann Nucl Med. 2022. PMID: 36214955
-
Contribution of Tumor-Derived Extracellular Vesicles to Malignant Transformation of Normal Cells.Bioengineering (Basel). 2022 Jun 4;9(6):245. doi: 10.3390/bioengineering9060245. Bioengineering (Basel). 2022. PMID: 35735488 Free PMC article. Review.
-
Extracellular vesicle mimetics engineered from mesenchymal stem cells and curcumin promote fibrosis regression in a mouse model of thioacetamide-induced liver fibrosis.Regen Ther. 2024 Oct 21;26:911-921. doi: 10.1016/j.reth.2024.10.005. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39502438 Free PMC article.
-
Role of Extracellular Vesicles in Thyroid Physiology and Diseases: Implications for Diagnosis and Treatment.Biomedicines. 2022 Oct 15;10(10):2585. doi: 10.3390/biomedicines10102585. Biomedicines. 2022. PMID: 36289847 Free PMC article. Review.
-
Radioiodine labeling and in vivo trafficking of extracellular vesicles.Sci Rep. 2021 Mar 3;11(1):5041. doi: 10.1038/s41598-021-84636-5. Sci Rep. 2021. PMID: 33658566 Free PMC article.
References
-
- Dohán O, De La Vieja A, Paroder V, et al. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24(1):48–77. - PubMed
-
- Oh JM, Kalimuthu S, Gangadaran P, et al. Reverting iodine avidity of radioactive-iodine refractory thyroid cancer with a new tyrosine kinase inhibitor (K905-0266) excavated by high-throughput NIS (sodium iodide symporter) enhancer screening platform using dual reporter gene system. Oncotarget. 2018;9(6):7075–7087. - PMC - PubMed
-
- Lee YL, Lee YJ, Ahn SJ, et al. Combined radionuclide-chemotherapy and in vivo imaging of hepatocellular carcinoma cells after transfection of a triple-gene construct, NIS, HSV1-sr39tk, and EGFP. Cancer Lett. 2010;290(1):129–138. - PubMed
-
- Seidlin SM, Marinelli LD, Oshry E. Radioactive iodine therapy: effect on functioning metastases of adenocarcinoma of the thyroid. CA Cancer J Clin. 1990;40(5):299–317. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

