Ubiquitin-specific peptidase 28 enhances STAT3 signaling and promotes cell growth in non-small-cell lung cancer
- PMID: 30881015
- PMCID: PMC6396656
- DOI: 10.2147/OTT.S194917
Ubiquitin-specific peptidase 28 enhances STAT3 signaling and promotes cell growth in non-small-cell lung cancer
Abstract
Background and objectives: Ubiquitin-specific peptidase 28 (USP28) has been reported to play significant roles in several tumors, but its roles in non-small-cell lung cancer (NSCLC) is still unknown. In this study, we aimed to investigate the biological function and molecular mechanisms of USP28 in NSCLC.
Materials and methods: Immunoblotting analysis was used to detect relative proteins' expression. Luciferase assay was performed to explore the activation of signal transducer and activator of transcription 3 (STAT3). Immunoprecipitation was performed to assess whether USP28 interacted with STAT3 or deubiquitinated STAT3. Quantitative real-time PCR was performed to evaluate the relative mRNA levels of STAT3 and USP28. Cycloheximide chase assay was carried out to examine whether USP28 affected the half-life of STAT3 protein. Cell Counting Kit-8 assay and xenograft model were used to assess whether USP28 regulated NSCLC cell growth.
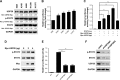
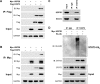
Results: In this study, the deubiquitinating enzyme USP28 was found to mediate STAT3 signaling in NSCLC cells. USP28 interacted with STAT3, and increased the stability of STAT3 by inducing its deubiquitination. Further studies showed that USP28 was upregulated in both the primary tissues and cell lines of NSCLC. The Kaplan-Meier plotter also indicated that USP28 predicted a poor prognosis of NSCLC patients. Moreover, knockdown of USP28 inhibited cell growth of NSCLC cells in vitro and delayed NSCLC tumor growth in vivo.
Conclusion: These results demonstrated that USP28 was functional in NSCLC cells, and promoted NSCLC cell growth by inducing STAT3 signaling. This suggests that USP28 could be a novel target for NSCLC therapy.
Keywords: STAT3; USP28; deubiquitinating enzyme; deubiquitination; non-small-cell lung cancer.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Overexpression of deubiquitinating enzyme USP28 promoted non-small cell lung cancer growth.J Cell Mol Med. 2015 Apr;19(4):799-805. doi: 10.1111/jcmm.12426. Epub 2015 Feb 5. J Cell Mol Med. 2015. PMID: 25656529 Free PMC article.
-
Long Non-coding RNA LINC01426 Contributes to the Malignant Behaviors of NSCLC Via Acting As a Sponge for miR-143-3p.Biochem Genet. 2022 Dec;60(6):2570-2586. doi: 10.1007/s10528-022-10234-3. Epub 2022 May 31. Biochem Genet. 2022. PMID: 35639219
-
miR-3940-5p Functions as a Tumor Suppressor in Non-Small Cell Lung Cancer Cells by Targeting Cyclin D1 and Ubiquitin Specific Peptidase-28.Transl Oncol. 2017 Feb;10(1):80-89. doi: 10.1016/j.tranon.2016.11.004. Epub 2016 Dec 15. Transl Oncol. 2017. PMID: 27988424 Free PMC article.
-
Circ_ZNF124 promotes non-small cell lung cancer progression by abolishing miR-337-3p mediated downregulation of JAK2/STAT3 signaling pathway.Cancer Cell Int. 2019 Nov 14;19:291. doi: 10.1186/s12935-019-1011-y. eCollection 2019. Cancer Cell Int. 2019. PMID: 31754348 Free PMC article.
-
Ubiquitin-specific protease 28: the decipherment of its dual roles in cancer development.Exp Hematol Oncol. 2023 Mar 6;12(1):27. doi: 10.1186/s40164-023-00389-z. Exp Hematol Oncol. 2023. PMID: 36879346 Free PMC article. Review.
Cited by
-
USP28: Oncogene or Tumor Suppressor? A Unifying Paradigm for Squamous Cell Carcinoma.Cells. 2021 Oct 4;10(10):2652. doi: 10.3390/cells10102652. Cells. 2021. PMID: 34685632 Free PMC article. Review.
-
Ubiquitin-specific protease 25 ameliorates ulcerative colitis by regulating the degradation of phosphor-STAT3.Cell Death Dis. 2025 Jan 7;16(1):5. doi: 10.1038/s41419-024-07315-z. Cell Death Dis. 2025. PMID: 39773987 Free PMC article.
-
Hepatocyte Apolipoprotein J Accelerates Injury-induced Liver Fibrosis by Activation Signal Transducer and Activator of Transcription 3 Through Ranbp2 Mediated-SUMOylation.Cell Mol Gastroenterol Hepatol. 2025 Jun 20;19(10):101556. doi: 10.1016/j.jcmgh.2025.101556. Online ahead of print. Cell Mol Gastroenterol Hepatol. 2025. PMID: 40545037 Free PMC article.
-
Targeted therapy based on ubiquitin-specific proteases, signalling pathways and E3 ligases in non-small-cell lung cancer.Front Oncol. 2023 Mar 9;13:1120828. doi: 10.3389/fonc.2023.1120828. eCollection 2023. Front Oncol. 2023. PMID: 36969062 Free PMC article. Review.
-
USP28 facilitates pancreatic cancer progression through activation of Wnt/β-catenin pathway via stabilising FOXM1.Cell Death Dis. 2021 Sep 28;12(10):887. doi: 10.1038/s41419-021-04163-z. Cell Death Dis. 2021. PMID: 34584067 Free PMC article.
References
-
- Komander D, Clague MJ, Urbé S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10(8):550–563. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

