A meta-analysis of the efficacy and safety of PD-1/PD-L1 immune checkpoint inhibitors as treatments for metastatic bladder cancer
- PMID: 30881032
- PMCID: PMC6404681
- DOI: 10.2147/OTT.S186271
A meta-analysis of the efficacy and safety of PD-1/PD-L1 immune checkpoint inhibitors as treatments for metastatic bladder cancer
Abstract
Background: This article is a meta-analysis aiming to systematically assess the efficacy and safety profiles of PD-1/PD-L1 inhibitors in patients with advanced or metastatic bladder cancer.
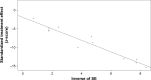
Methods: We extracted and examined data from phase I, II, and III clinical trials from the Medline, Embase, and the Cochrane Library, which included patients with metastatic bladder cancer who were treated with PD-1/PD-L1 inhibitors. We performed a meta-analysis to investigate several indexes of efficacy and safety, including the objective response rate (ORR), 1-year overall survival (OS) rate, 1-year progression-free survival (PFS) rate, and adverse event (AE) rate of immune checkpoint inhibitors. The material data were calculated and pooled using The R Project for Statistical Computing and Review Manager 5.3.
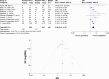
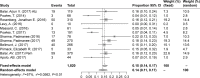
Results: After excluding ineligible records, 14 clinical trials were included in our analysis. The pooled frequencies of all-grade AEs and grade ≥3 AEs were 0.63 (95% CI 0.61-0.65, P=0.34) and 0.14 (95% CI 0.11-0.17, P=0.0072), respectively. The summary ORR was 0.21 (95% CI 0.18-0.24 P=0.07), and the 1-year OS and 1-year PFS rates were 0.48 (95% CI 0.42-0.54 P=0.0013) and 0.21 (95% CI 0.16-0.26 P=0.04), respectively. The OR of ORR between the PD-L1-positive and -negative groups was 3.09 (95% CI 2.01-4.75, P=0.08).
Conclusion: The PD-1/PD-L1 therapy showed appropriate efficacy and acceptable incidence of treatment-related AEs. In addition, the level of discrimination of PD-L1 expression might be related to the effect of the PD-1/PD-L1 inhibitors, and patients displaying positive expression might experience a better curative effect than patients displaying negative expression.
Keywords: PD-1 inhibitor; PD-L1 inhibitor; bladder cancer; immunotherapy; meta-analysis; metastatic bladder cancer; oncology.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures






References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2015;60(5):277–300. - PubMed
-
- Bellmunt J, Orsola A, Wiegel T, et al. Bladder cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi45–vi49. - PubMed
-
- Milowsky MI, Rumble RB, Booth CM, et al. Guideline on muscle-invasive and metastatic bladder cancer (European Association of Urology Guideline): American society of clinical oncology clinical practice guideline endorsement. J Clin Oncol. 2016;34(16):1945–1952. - PubMed
-
- Wong YNS, Joshi K, Pule M, et al. Evolving adoptive cellular therapies in urological malignancies. Lancet Oncol. 2017;18(6):e341. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

