APOLLO-1: a randomized placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the µ-opioid receptor, for management of moderate-to-severe acute pain following bunionectomy
- PMID: 30881102
- PMCID: PMC6417853
- DOI: 10.2147/JPR.S171013
APOLLO-1: a randomized placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the µ-opioid receptor, for management of moderate-to-severe acute pain following bunionectomy
Abstract
Purpose: Oliceridine is a novel G protein-biased µ-opioid receptor agonist designed to provide intravenous (IV) analgesia with a lower risk of opioid-related adverse events (ORAEs) than conventional opioids.
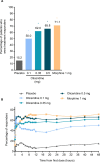
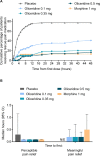
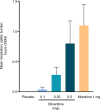
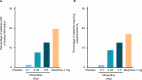
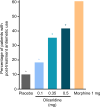
Patients and methods: APOLLO-1 (NCT02815709) was a phase III, double-blind, randomized trial in patients with moderate-to-severe pain following bunionectomy. Patients received a loading dose of either placebo, oliceridine (1.5 mg), or morphine (4 mg), followed by demand doses via patient-controlled analgesia (0.1, 0.35, or 0.5 mg oliceridine, 1 mg morphine, or placebo). The primary endpoint compared the proportion of treatment responders through 48 hours for oliceridine regimens and placebo. Secondary outcomes included a composite measure of respiratory safety burden (RSB, representing the cumulative duration of respiratory safety events) and the proportion of treatment responders vs morphine.
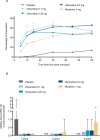
Results: Effective analgesia was observed for all oliceridine regimens, with responder rates of 50%, 62%, and 65.8% in the 0.1 mg, 0.35 mg, and 0.5 mg regimens, respectively (all P<0.0001 vs placebo [15.2%]; 0.35 mg and 0.5 mg non-inferior to morphine). RSB showed a dose-dependent increase across oliceridine regimens (mean hours [SD]: 0.1 mg: 0.04 [0.33]; 0.35 mg: 0.28 [1.11]; 0.5 mg: 0.8 [3.33]; placebo: 0 [0]), but none were statistically different from morphine (1.1 [3.03]). Gastrointestinal adverse events also increased in a dose-dependent manner in oliceridine regimens (0.1 mg: 40.8%; 0.35 mg: 59.5%; 0.5 mg: 70.9%; placebo: 24.1%; morphine: 72.4%). The odds ratio for rescue antiemetic use was significantly lower for oliceridine regimens compared to morphine (P<0.05).
Conclusion: Oliceridine is a novel and effective IV analgesic providing rapid analgesia for the relief of moderate-to-severe acute postoperative pain compared to placebo. Additionally, it has a favorable safety and tolerability profile with regard to respiratory and gastrointestinal adverse effects compared to morphine, and may provide a new treatment option for patients with moderate-to-severe postoperative pain where an IV opioid is required.
Keywords: analgesia; clinical trial; orthopedic surgery; patient controlled; postoperative.
Conflict of interest statement
Disclosure David A Burt, Emily Cook, David G Soergel, and Franck Skobieranda were full-time employees and stockholders of Trevena Inc. at the time the research was conducted and manuscript was under preparation. Neil Singla is founder and CEO of Lotus Clinical Research, LLC, an analgesic Clinical Research Organization and research site; in this capacity he has served as a consultant and provided clinical trial services for Trevena Inc. Eugene R Viscusi has served as a consultant to Trevena Inc. The authors report no other conflicts of interest in this work.
Figures
References
-
- National Pharmaceutical Council (NPC) Pain: current understanding of assessment, management, and treatments. Reston, VA: NPC, Joint Commission on Accreditation of Health Care Organization; 2001.
-
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–S120. - PubMed
-
- Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159–180. - PubMed
-
- Swegle JM, Logemann C. Management of common opioid-induced adverse effects. Am Fam Physician. 2006;74(8):1347–1354. - PubMed
LinkOut - more resources
Full Text Sources
Medical

