Alzheimer's Disease and Cancer: When Two Monsters Cannot Be Together
- PMID: 30881282
- PMCID: PMC6407038
- DOI: 10.3389/fnins.2019.00155
Alzheimer's Disease and Cancer: When Two Monsters Cannot Be Together
Abstract
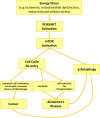
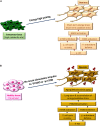
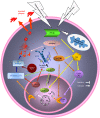
Alzheimer's disease (AD) and cancer are among the leading causes of human death around the world. While neurodegeneration is the main feature of AD, the most important characteristic of malignant tumors is cell proliferation, placing these two diseases in opposite sides of cell division spectrum. Interestingly, AD and cancer's pathologies consist of a remarkable common feature and that is the presence of active cell cycle in both conditions. In an in vitro model of primary adult neuronal culture, we previously showed that treating cell with beta amyloid forced neurons to start a cell cycle. Instead of cell division, however, neuronal cell cycle was aborted and a massive neurodegeneration was left behind as the consequence. A high level of cell cycle entry, which is a requirement for cancer pathogenesis, was reported in clinically diagnosed cases of AD, leading to neurodegeneration. The diverse clinical manifestation of a similar etiology, have puzzled researchers for many years. In fact, the evidence showed an inverse association between AD and cancer prevalence, suggesting that switching pathogenesis toward AD protects patients against cancer and vice versa. In this mini review, we discussed the possibility of involvement of cell proliferation and survival dysregulation as the underlying mechanism of neurodegeneration in AD, and the leading event to develop both disorders' pathology. As examples, the role of phosphoinositide 3 kinase/Akt/ mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway in cell cycle re-entry and blocking autophagy are discussed as potential common intracellular components between AD and cancer pathogenesis, with diverse clinical diagnosis.
Keywords: Alzheimer’s disease; PI3K/Akt/mTOR; autophagy; beta amyloid; cancer; cell cycle; neurodegeneration; tau phosphorylation.
Figures
Similar articles
-
mTORC2 (Rictor) in Alzheimer's Disease and Reversal of Amyloid-β Expression-Induced Insulin Resistance and Toxicity in Rat Primary Cortical Neurons.J Alzheimers Dis. 2017;56(3):1015-1036. doi: 10.3233/JAD-161029. J Alzheimers Dis. 2017. PMID: 28035937 Free PMC article.
-
[Moxibustion at acpoints of governor vessel on regulating PI3K/Akt/mTOR signaling pathway and enhancing autophagy process in APP/PS1 double-transgenic Alzheimer's disease mice].Zhongguo Zhen Jiu. 2019 Dec 12;39(12):1313-8. doi: 10.13703/j.0255-2930.2019.12.015. Zhongguo Zhen Jiu. 2019. PMID: 31820607 Chinese.
-
Activation of mTOR: a culprit of Alzheimer's disease?Neuropsychiatr Dis Treat. 2015 Apr 9;11:1015-30. doi: 10.2147/NDT.S75717. eCollection 2015. Neuropsychiatr Dis Treat. 2015. PMID: 25914534 Free PMC article. Review.
-
Selenomethionine Mitigates Cognitive Decline by Targeting Both Tau Hyperphosphorylation and Autophagic Clearance in an Alzheimer's Disease Mouse Model.J Neurosci. 2017 Mar 1;37(9):2449-2462. doi: 10.1523/JNEUROSCI.3229-16.2017. Epub 2017 Jan 30. J Neurosci. 2017. PMID: 28137967 Free PMC article.
-
A Unified Hypothesis of Early- and Late-Onset Alzheimer's Disease Pathogenesis.J Alzheimers Dis. 2015;47(1):33-47. doi: 10.3233/JAD-143210. J Alzheimers Dis. 2015. PMID: 26402752 Free PMC article. Review.
Cited by
-
Gene expression data analysis using Hellinger correlation in weighted gene co-expression networks (WGCNA).Comput Struct Biotechnol J. 2022 Jul 13;20:3851-3863. doi: 10.1016/j.csbj.2022.07.018. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35891798 Free PMC article.
-
c-Myc and FOXO3a-The Everlasting Decision Between Neural Regeneration and Degeneration.Int J Mol Sci. 2024 Nov 24;25(23):12621. doi: 10.3390/ijms252312621. Int J Mol Sci. 2024. PMID: 39684331 Free PMC article. Review.
-
Pain as a Protective Factor for Alzheimer Disease in Patients with Cancer.Cancers (Basel). 2022 Dec 30;15(1):248. doi: 10.3390/cancers15010248. Cancers (Basel). 2022. PMID: 36612244 Free PMC article.
-
Regulation of the Soluble Amyloid Precursor Protein α (sAPPα) Levels by Acetylcholinesterase and Brain-Derived Neurotrophic Factor in Lung Cancer Cell Media.Int J Mol Sci. 2022 Sep 15;23(18):10746. doi: 10.3390/ijms231810746. Int J Mol Sci. 2022. PMID: 36142659 Free PMC article.
-
Inverse Correlation Between Alzheimer's Disease and Cancer: Short Overview.Mol Neurobiol. 2021 Dec;58(12):6335-6349. doi: 10.1007/s12035-021-02544-1. Epub 2021 Sep 14. Mol Neurobiol. 2021. PMID: 34523079 Free PMC article. Review.
References
-
- Alzheimer (1906). Über einen eigenartigen schweren Erkrankungsprozeβ der Hirnrincle. Neurol. Central 25:1134.
-
- Arendt T. (2003). Synaptic plasticity and cell cycle activation in neurons are alternative effector pathways: the ’Dr. Jekyll and Mr. Hyde concept’ of Alzheimer’s disease or the yin and yang of neuroplasticity. Prog. Neurobiol. 71 83–248. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

