Neuroglial Involvement in Abnormal Glutamate Transport in the Cochlear Nuclei of the Igf1 -/- Mouse
- PMID: 30881288
- PMCID: PMC6405628
- DOI: 10.3389/fncel.2019.00067
Neuroglial Involvement in Abnormal Glutamate Transport in the Cochlear Nuclei of the Igf1 -/- Mouse
Abstract
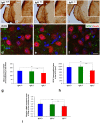
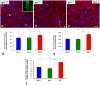
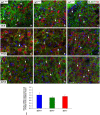
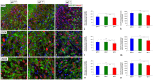
Insulin-like growth factor 1 (IGF-1) is a powerful regulator of synaptic activity and a deficit in this protein has a profound impact on neurotransmission, mostly on excitatory synapses in both the developing and mature auditory system. Adult Igf1 -/- mice are animal models for the study of human syndromic deafness; they show altered cochlear projection patterns into abnormally developed auditory neurons along with impaired glutamate uptake in the cochlear nuclei, phenomena that probably reflect disruptions in neuronal circuits. To determine the cellular mechanisms that might be involved in regulating excitatory synaptic plasticity in 4-month-old Igf1 -/- mice, modifications to neuroglia, astroglial glutamate transporters (GLTs) and metabotropic glutamate receptors (mGluRs) were assessed in the cochlear nuclei. The Igf1 -/- mice show significant decreases in IBA1 (an ionized calcium-binding adapter) and glial fibrillary acidic protein (GFAP) mRNA expression and protein accumulation, as well as dampened mGluR expression in conjunction with enhanced glutamate transporter 1 (GLT1) expression. By contrast, no differences were observed in the expression of glutamate aspartate transporter (GLAST) between these Igf1 -/- mice and their heterozygous or wildtype littermates. These observations suggest that congenital IGF-1 deficiency may lead to alterations in microglia and astrocytes, an upregulation of GLT1, and the downregulation of groups I, II and III mGluRs. Understanding the molecular, biochemical and morphological mechanisms underlying neuronal plasticity in a mouse model of hearing deficits will give us insight into new therapeutic strategies that could help to maintain or even improve residual hearing when human deafness is related to IGF-1 deficiency.
Keywords: IGF-1; astrocytes; cochlear nucleus; glutamate receptors; hearing loss; microglia.
Figures







Similar articles
-
IGF-1 deficiency causes atrophic changes associated with upregulation of VGluT1 and downregulation of MEF2 transcription factors in the mouse cochlear nuclei.Brain Struct Funct. 2016 Mar;221(2):709-34. doi: 10.1007/s00429-014-0934-2. Epub 2014 Nov 7. Brain Struct Funct. 2016. PMID: 25378055
-
Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons.J Neurosci. 2004 May 12;24(19):4551-9. doi: 10.1523/JNEUROSCI.5217-03.2004. J Neurosci. 2004. PMID: 15140926 Free PMC article.
-
The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms.Neurochem Int. 2000 Aug-Sep;37(2-3):163-70. doi: 10.1016/s0197-0186(00)00019-x. Neurochem Int. 2000. PMID: 10812201
-
Control of the phosphorylation of the astrocyte marker glial fibrillary acidic protein (GFAP) in the immature rat hippocampus by glutamate and calcium ions: possible key factor in astrocytic plasticity.Braz J Med Biol Res. 1997 Mar;30(3):325-38. doi: 10.1590/s0100-879x1997000300005. Braz J Med Biol Res. 1997. PMID: 9246230 Review.
-
[Role of glutamate transporters in excitatory synapses in cerebellar Purkinje cells].Brain Nerve. 2007 Jul;59(7):669-76. Brain Nerve. 2007. PMID: 17663137 Review. Japanese.
Cited by
-
IGF-1 Haploinsufficiency Causes Age-Related Chronic Cochlear Inflammation and Increases Noise-Induced Hearing Loss.Cells. 2021 Jul 3;10(7):1686. doi: 10.3390/cells10071686. Cells. 2021. PMID: 34359856 Free PMC article.
-
Age-Related Inflammation and Oxidative Stress in the Cochlea Are Exacerbated by Long-Term, Short-Duration Noise Stimulation.Front Aging Neurosci. 2022 Apr 5;14:853320. doi: 10.3389/fnagi.2022.853320. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35450058 Free PMC article.
-
Insulin-like growth factor 1 partially rescues early developmental defects caused by SHANK2 knockdown in human neurons.Neural Regen Res. 2020 Dec;15(12):2335-2343. doi: 10.4103/1673-5374.285002. Neural Regen Res. 2020. PMID: 32594058 Free PMC article.
-
Modulating Microglial Cells for Promoting Brain Recovery and Repair.Front Cell Neurosci. 2021 Jan 11;14:627987. doi: 10.3389/fncel.2020.627987. eCollection 2020. Front Cell Neurosci. 2021. PMID: 33505251 Free PMC article.
-
In vivo Bioluminescence Imaging Used to Monitor Disease Activity and Therapeutic Response in a Mouse Model of Tauopathy.Front Aging Neurosci. 2019 Sep 12;11:252. doi: 10.3389/fnagi.2019.00252. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31572168 Free PMC article.
References
-
- Alvarado J. C., Fuentes-Santamaría V., Franklin S. R., Brunso-Bechtold J. K., Henkel C. K. (2007a). Synaptophysin and insulin-like growth factor-1 immunostaining in the central nucleus of the inferior colliculus in adult ferrets following unilateral cochlear removal: a densitometric analysis. Synapse 61, 288–302. 10.1002/syn.20373 - DOI - PubMed

