TRP Channels: Current Perspectives in the Adverse Cardiac Remodeling
- PMID: 30881310
- PMCID: PMC6406032
- DOI: 10.3389/fphys.2019.00159
TRP Channels: Current Perspectives in the Adverse Cardiac Remodeling
Abstract
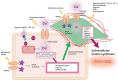
Calcium is an important second messenger required not only for the excitation-contraction coupling of the heart but also critical for the activation of cell signaling pathways involved in the adverse cardiac remodeling and consequently for the heart failure. Sustained neurohumoral activation, pressure-overload, or myocardial injury can cause pathologic hypertrophic growth of the heart followed by interstitial fibrosis. The consequent heart's structural and molecular adaptation might elevate the risk of developing heart failure and malignant arrhythmia. Compelling evidences have demonstrated that Ca2+ entry through TRP channels might play pivotal roles in cardiac function and pathology. TRP proteins are classified into six subfamilies: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPML (mucolipin), and TRPP (polycystin), which are activated by numerous physical and/or chemical stimuli. TRP channels participate to the handling of the intracellular Ca2+ concentration in cardiac myocytes and are mediators of different cardiovascular alterations. This review provides an overview of the current knowledge of TRP proteins implication in the pathologic process of some frequent cardiac diseases associated with the adverse cardiac remodeling such as cardiac hypertrophy, fibrosis, and conduction alteration.
Keywords: TRP channels; calcium; cardiac remodeling; conduction disorders; fibrosis; hypertrophy.
Figures

References
-
- Adapala R. K., Thoppil R. J., Luther D. J., Paruchuri S., Meszaros J. G., Chilian W. M., et al. . (2013). TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J. Mol. Cell. Cardiol. 54, 45–52. 10.1016/j.yjmcc.2012.10.016, PMID: - DOI - PMC - PubMed
-
- Adapala R., Minasyan A., Kanugula A. K., Cappelli H. C., Paruchuri S., Meszaros G. J., et al. (2018). Targeting TRPV4 channels protects heart from pathological remodeling following myocardial infarction. Circulation. 136:A24061.
-
- Avila-Medina J., Mayoral-Gonzalez I., Dominguez-Rodriguez A., Gallardo-Castillo I., Ribas J., Ordoñez A., et al. . (2018). The complex role of store operated calcium entry pathways and related proteins in the function of cardiac, skeletal and vascular smooth muscle cells. Front. Physiol. 9:257. 10.3389/fphys.2018.00257, PMID: - DOI - PMC - PubMed

