Spinal Cord Glycine Transporter 2 Mediates Bilateral ST35 Acupoints Sensitization in Rats with Knee Osteoarthritis
- PMID: 30881475
- PMCID: PMC6383421
- DOI: 10.1155/2019/7493286
Spinal Cord Glycine Transporter 2 Mediates Bilateral ST35 Acupoints Sensitization in Rats with Knee Osteoarthritis
Abstract
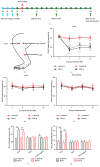
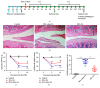
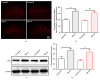
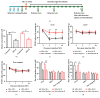
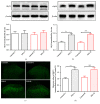
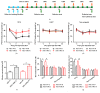
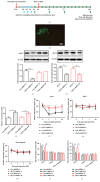
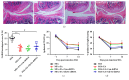
The concept of "acupoint sensitization" refers to the functional status of acupoint switches from silent to active under pathological conditions. In clinic, acupoint sensitization provides important guidance for acupoints selection in different diseases. However, the mechanism behind this phenomenon remains unclear. We generated a model of knee osteoarthritis (KOA) by intra-articular injection of monosodium iodoacetate (MIA) into the left knee of rats. The paw withdrawal mechanical threshold (PWMT) and the total number of mast cells as well as mast cell degranulation rate (MCDR) of acupoint tissue were used to test whether the acupoints were sensitized. The results showed that KOA resulted in a reduced mechanical threshold and elevated total number of mast cell as well as mast cell degranulation rate at bilateral ST35 (Dubi) but not GB37 (Guangming) or nonacupoint area. The acupoint sensitization was accompanied by upregulation of glycine transporter 2 (GlyT2) and reduction of extracellular glycine levels in the bilateral dorsal horns of the spinal cord at L3-5. Selective inhibition of GlyT2 or intrathecal administration of glycine attenuated ST35 acupoint sensitization. The sensitization of bilateral ST35 was blocked after intraspinal GlyT2 short hairpin (sh) RNA (GlyT2-shRNA) microinjection to specifically downregulate GlyT2 expression in the left side (ipsilateral) L3-5 spinal cord dorsal horn before MIA injection. Moreover, electroacupuncture (EA) stimulation at ST35 ameliorated articular pathological lesions and improved KOA-related pain behaviors. GlyT2-shRNA injection reversed EA-induced pain relief but not EA-induced reduction of joint lesions. Overall, this study demonstrated that spinal GlyT2, especially elevated GlyT2 expression in the ipsilateral dorsal horn of the spinal cord, is a crucial mediator of ST35 acupoint sensitization in KOA rats.
Figures
Similar articles
-
Laser Moxibustion Alleviates Knee Osteoarthritis Pain by Inhibiting Spinal Microglial Activation-Mediated Neuroinflammation in Rats.Photobiomodul Photomed Laser Surg. 2020 Apr;38(4):237-243. doi: 10.1089/photob.2019.4744. Epub 2020 Jan 23. Photobiomodul Photomed Laser Surg. 2020. PMID: 31976816
-
Acupoint Sensitization is Associated with Increased Excitability and Hyperpolarization-Activated Current (Ih) in C- But Not Aδ-Type Neurons.Neuroscience. 2019 Apr 15;404:499-509. doi: 10.1016/j.neuroscience.2019.02.028. Epub 2019 Mar 1. Neuroscience. 2019. PMID: 30826524
-
[Effect of electroacupuncture on the expression of Iba-1 in spinal dorsal horn and hippocampus of chronic pain rats with knee osteoarthritis].Zhen Ci Yan Jiu. 2023 May 25;48(5):431-7. doi: 10.13702/j.1000-0607.20211398. Zhen Ci Yan Jiu. 2023. PMID: 37247855 Chinese.
-
[Complex network analysis on regularities of acupoint combinations and application characteristics of acupuncture and moxibustion in the treatment of knee osteoarthritis].Zhen Ci Yan Jiu. 2022 Jan 25;47(1):65-70. doi: 10.13702/j.1000-0607.20210080. Zhen Ci Yan Jiu. 2022. PMID: 35128873 Review. Chinese.
-
[Roles of nociceptors in acupoint sensitization: recent advances].Zhen Ci Yan Jiu. 2021 Dec 25;46(12):1048-56. doi: 10.13702/j.1000-0607.20210313. Zhen Ci Yan Jiu. 2021. PMID: 34970883 Review. Chinese.
Cited by
-
Acupuncture Enhances Signals at Sensitized Acupoints to Elevate Pressure Pain Threshold in Knee Osteoarthritis Patients.Chin J Integr Med. 2022 Dec;28(12):1105-1110. doi: 10.1007/s11655-022-3588-6. Epub 2022 Nov 22. Chin J Integr Med. 2022. PMID: 36417139
-
Acupuncture modulates extracellular ATP levels in peripheral sensory nervous system during analgesia of ankle arthritis in rats.Purinergic Signal. 2021 Sep;17(3):411-424. doi: 10.1007/s11302-021-09777-8. Epub 2021 May 2. Purinergic Signal. 2021. PMID: 33934245 Free PMC article.
-
Effect of Moxibustion on the Intestinal Flora of Rats with Knee Osteoarthritis Induced by Monosodium Iodoacetate.Evid Based Complement Alternat Med. 2020 Jul 4;2020:3196427. doi: 10.1155/2020/3196427. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32714401 Free PMC article.
-
The Effects of Laser Moxibustion on Knee Osteoarthritis Pain in Rats.Photobiomodul Photomed Laser Surg. 2020 Jan;38(1):43-50. doi: 10.1089/photob.2019.4716. Epub 2019 Sep 24. Photobiomodul Photomed Laser Surg. 2020. PMID: 31549920 Free PMC article.
-
Inhibition of Glycine Re-Uptake: A Potential Approach for Treating Pain by Augmenting Glycine-Mediated Spinal Neurotransmission and Blunting Central Nociceptive Signaling.Biomolecules. 2021 Jun 10;11(6):864. doi: 10.3390/biom11060864. Biomolecules. 2021. PMID: 34200954 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

