Aromatic C-H amination in hexafluoroisopropanol
- PMID: 30881670
- PMCID: PMC6385662
- DOI: 10.1039/c8sc04966a
Aromatic C-H amination in hexafluoroisopropanol
Abstract
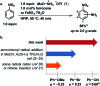
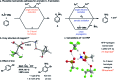
We report a direct radical aromatic amination reaction that provides unprotected anilines with an improvement in the substrate scope compared to prior art. Hydrogen bonding by the solvent hexafluoroisopropanol to anions of cationic species is responsible for increased reactivity and can rationalize the enhancement in substrate scope. Our findings may have bearings on radical additions to arenes for direct C-H functionalization in general.
Figures
References
-
- Kuhl N., Hopkinson M. N., Wencel-Delord J., Glorius F. Angew. Chem., Int. Ed. 2012;51:10236–10254. - PubMed
-
- Minisci F. Synthesis. 1973:1–24.
-
- Bolton R., Williams G. H. Chem. Soc. Rev. 1986;15:261–289.
-
- Studer A. Angew. Chem., Int. Ed. 2012;51:8950–8958. - PubMed
-
- Rossi R., Budén M. E. and Guastavino J. F., in Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds, ed. J. Mortier, John Wiley & Sons, Hoboken, 2016, pp. 219–242.
LinkOut - more resources
Full Text Sources

