Autotaxin and Lipoprotein Metabolism in Calcific Aortic Valve Disease
- PMID: 30881959
- PMCID: PMC6405425
- DOI: 10.3389/fcvm.2019.00018
Autotaxin and Lipoprotein Metabolism in Calcific Aortic Valve Disease
Abstract
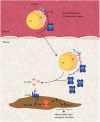
Calcific aortic valve disease (CAVD) is a complex trait disorder characterized by calcific remodeling of leaflets. Genome-wide association (GWA) study and Mendelian randomization (MR) have highlighted that LPA, which encodes for apolipoprotein(a) [apo(a)], is causally associated with CAVD. Apo(a) is the protein component of Lp(a), a LDL-like particle, which transports oxidized phospholipids (OxPLs). Autotaxin (ATX), which is encoded by ENPP2, is a member of the ecto-nucleotidase family of enzymes, which is, however, a lysophospholipase. As such, ATX converts phospholipids into lysophosphatidic acid (LysoPA), a metabolite with potent and diverse biological properties. Studies have recently underlined that ATX is enriched in the Lp(a) lipid fraction. Functional experiments and data obtained in mouse models suggest that ATX mediates inflammation and mineralization of the aortic valve. Recent findings also indicate that epigenetically-driven processes lower the expression of phospholipid phosphatase 3 (PLPP3) and increased LysoPA signaling and inflammation in the aortic valve during CAVD. These recent data thus provide novel insights about how lipoproteins mediate the development of CAVD. Herein, we review the implication of lipoproteins in CAVD and examine the role of ATX in promoting the osteogenic transition of valve interstitial cells (VICs).
Keywords: aortic valve; autotaxin (ATX); calcific aortic stenosis; inflammation; phospholipid (PL).
Figures

References
-
- Yu Z, Seya K, Daitoku K, Motomura S, Fukuda I, Furukawa K. Tumor necrosis factor-alpha accelerates the calcification of human aortic valve interstitial cells obtained from patients with calcific aortic valve stenosis via the BMP2-Dlx5 pathway. J Pharmacol Exp Ther. (2011) 337:16–23. 10.1124/jpet.110.177915 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

