Real-world persistence and benefit-risk profile of fingolimod over 36 months in Germany
- PMID: 30882022
- PMCID: PMC6410931
- DOI: 10.1212/NXI.0000000000000548
Real-world persistence and benefit-risk profile of fingolimod over 36 months in Germany
Abstract
Objective: To assess the long-term real-world benefit-risk profile of fingolimod in patients with relapsing MS in Germany.
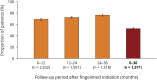
Methods: This analysis used data from the noninterventional real-world study, Post-Authorization Non-interventional German sAfety study of GilEnyA (PANGAEA), to assess prospectively the persistence, effectiveness, and safety of fingolimod over 36 months (±90 days) in Germany. For inclusion in the effectiveness analysis (n = 2,537), patients were required to have received fingolimod for the first time in PANGAEA, to have at least 12 months of data, and to have completed each 12-month follow-up period. For the safety analysis (n = 3,266), patients were additionally allowed to have received fingolimod before enrollment.
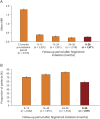
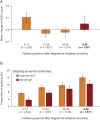
Results: At baseline, 94.7% of patients in the effectiveness analysis had received a previous disease-modifying therapy. After 36 months, 70.4% of patients were still receiving fingolimod. Over this period, annualized relapse rates decreased to 0.265 (95% CI: 0.244-0.286) from 1.79 (95% CI: 1.75-1.83), and mean Expanded Disability Status Scale scores remained stable (mean change from baseline: +0.049 [95% CI: -0.015 to +0.114]). In total, 16% of patients had 6-month confirmed disability improvement, 12.5% had 6-month confirmed disability worsening, and 52.4% were free from relapses and 6-month confirmed disability worsening. Adverse events (AEs) and serious AEs were experienced by up to 23.4% and 3.9% of patients, respectively, during any of the 12-month follow-up periods. The frequency and nature of AEs were in line with previous findings.
Conclusions: Using systematically collected data from PANGAEA, this analysis demonstrates the sustained effectiveness, high persistence, and manageable safety profile of fingolimod over 36 months.
Figures
References
-
- Trojano M, Tintore M, Montalban X, et al. . Treatment decisions in multiple sclerosis—insights from real-world observational studies. Nat Rev Neurol 2017;13:105–118. - PubMed
-
- Ziemssen T, De Stefano N, Pia Sormani M, Van Wijmeersch B, Wiendl H, Kieseier BC. Optimizing therapy early in multiple sclerosis: an evidence-based view. Mult Scler Relat Disord 2015;4:460–469. - PubMed
-
- ABPI. Guidance—demonstrating value with real world data: a practical guide. 2011. Available at: abpi.org.uk/media/1591/2011-06-13-abpi-guidance-demonstrating-value-with.... Accessed December 20, 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
