Tuning the Excited-State Dynamics of CuI Films with Electrochemical Bias
- PMID: 30882041
- PMCID: PMC6413481
- DOI: 10.1021/acsenergylett.9b00182
Tuning the Excited-State Dynamics of CuI Films with Electrochemical Bias
Abstract
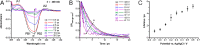
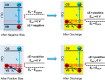
Owing to its high hole conductivity and ease of preparation, CuI was among the first inorganic hole-transporting materials that were introduced early on in metal halide perovskite solar cells, but its full potential as a semiconductor material is still to be realized. We have now performed ultrafast spectroelectrochemical experiments on ITO/CuI electrodes to show the effect of applied bias on the excited-state dynamics in CuI. Under operating conditions, the recombination of excitons is dependent on the applied bias, and it can be accelerated by decreasing the potential from +0.6 to -0.1 V vs Ag/AgCl. Prebiasing experiments show the persistent and reversible "memory" effect of electrochemical bias on charge carrier lifetimes. The excitation of CuI in a CuI/CsPbBr3 film provides synergy between both CuI and CsPbBr3 in dictating the charge separation and recombination.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Yang M.; Kim D. H.; Klein T. R.; Li Z.; Reese M. O.; Tremolet de Villers B. J.; Berry J. J.; van Hest M. F. A. M.; Zhu K. Highly Efficient Perovskite Solar Modules by Scalable Fabrication and Interconnection Optimization. ACS Energy Lett. 2018, 3 (2), 322–328. 10.1021/acsenergylett.7b01221. - DOI
-
- Hörantner M. T.; Leijtens T.; Ziffer M. E.; Eperon G. E.; Christoforo M. G.; McGehee M. D.; Snaith H. J. The Potential of Multijunction Perovskite Solar Cells. ACS Energy Lett. 2017, 2 (10), 2506–2513. 10.1021/acsenergylett.7b00647. - DOI
-
- Christians J. A.; Schulz P.; Tinkham J. S.; Schloemer T. H.; Harvey S. P.; Tremolet de Villers B. J.; Sellinger A.; Berry J. J.; Luther J. M. Tailored Interfaces of Unencapsulated Perovskite Solar Cells for > 1,000 h Operational Stability. Nat. Energy 2018, 3 (1), 68–74. 10.1038/s41560-017-0067-y. - DOI
-
- Schulz P. Interface Design for Metal Halide Perovskite Solar Cells. ACS Energy Lett. 2018, 3 (6), 1287–1293. 10.1021/acsenergylett.8b00404. - DOI
LinkOut - more resources
Full Text Sources
