Fish oil-based injectable lipid emulsions containing medium-chain triglycerides or added α-tocopherol offer anti-inflammatory benefits in a murine model of parenteral nutrition-induced liver injury
- PMID: 30882140
- PMCID: PMC6462433
- DOI: 10.1093/ajcn/nqy370
Fish oil-based injectable lipid emulsions containing medium-chain triglycerides or added α-tocopherol offer anti-inflammatory benefits in a murine model of parenteral nutrition-induced liver injury
Abstract
Background: Fish oil (FO) intravenous lipid emulsions (ILEs) are used as a monotherapy to treat parenteral nutrition (PN)-associated liver disease and provide essential fatty acids (EFAs) needed to sustain growth and prevent EFA deficiency (EFAD). Studies have suggested that medium-chain triglycerides (MCTs) and α-tocopherol have anti-inflammatory properties.
Objective: The purpose of this study was to test whether FO-ILEs containing MCTs and/or additional α-tocopherol decrease the inflammatory response to an endotoxin challenge compared with FO-ILE alone and preserve the ability to prevent PN-induced liver injury in mice.
Methods: A murine model of PN-induced hepatosteatosis was used to compare the effects of ILEs formulated in the laboratory containing varying ratios of FO and MCTs, and subsequently FO- and 50:50 FO:MCT-ILE plus 500 mg/L α-tocopherol (FO + AT and 50:50 + AT, respectively). C57BL/6 mice receiving unpurified diet (UPD), PN-equivalent diet (PN) + saline, and PN + soybean oil (SO)-ILE served as controls. After 19 d, mice received an intraperitoneal saline or endotoxin challenge 4 h before being killed. Serum and livers were harvested for histologic analysis, fatty acid profiling, and measurement of systemic inflammatory markers (tumor necrosis factor-α, interleukin-6).
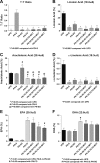
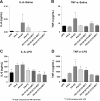
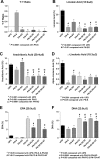
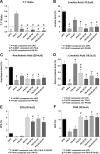
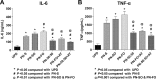
Results: All ILEs were well tolerated and prevented biochemical EFAD. Livers of mice that received saline and SO developed steatosis. Mice that received 30:70 FO:MCT developed mild hepatosteatosis. All other FO-containing ILEs preserved normal hepatic architecture. Mice that received FO- or SO-ILE had significantly elevated systemic inflammatory markers after endotoxin challenge compared with UPD-fed controls, whereas 50:50 FO:MCT, 30:70 FO:MCT, FO + AT, and 50:50 + AT groups had significantly lower inflammatory markers similar to those seen in UPD-fed controls.
Conclusions: Mixed FO/MCT and the addition of α-tocopherol to FO improved the inflammatory response to endotoxin challenge compared with FO-ILE alone while still preventing PN-induced liver injury and EFAD in mice. There was no synergistic relation between α-tocopherol and MCTs.
Keywords: fish oil; hepatosteatosis; injectable lipid emulsions; intestinal failure–associated liver disease; intravenous fat emulsions; medium-chain triglycerides; parenteral nutrition; parenteral nutrition–associated liver disease; α-tocopherol.
Copyright © American Society for Nutrition 2019.
Figures


Similar articles
-
Intestinal failure-associated liver disease model: a reduced phytosterol intravenous lipid emulsion prevents liver injury.Pediatr Res. 2025 Jun;97(7):2454-2461. doi: 10.1038/s41390-024-03753-9. Epub 2024 Nov 27. Pediatr Res. 2025. PMID: 39592772
-
Alpha-tocopherol in intravenous lipid emulsions imparts hepatic protection in a murine model of hepatosteatosis induced by the enteral administration of a parenteral nutrition solution.PLoS One. 2019 Jul 11;14(7):e0217155. doi: 10.1371/journal.pone.0217155. eCollection 2019. PLoS One. 2019. PMID: 31295333 Free PMC article.
-
Parenteral Soybean Oil Induces Hepatosteatosis Despite Addition of Fish Oil in a Mouse Model of Intestinal Failure-Associated Liver Disease.JPEN J Parenter Enteral Nutr. 2018 Feb;42(2):403-411. doi: 10.1177/0148607117695249. Epub 2017 Dec 13. JPEN J Parenter Enteral Nutr. 2018. PMID: 29187040
-
Intravenous Lipid Emulsions in the Prevention and Treatment of Liver Disease in Intestinal Failure.Nutrients. 2021 Mar 10;13(3):895. doi: 10.3390/nu13030895. Nutrients. 2021. PMID: 33801970 Free PMC article. Review.
-
Emergence of Mixed-Oil Fat Emulsions for Use in Parenteral Nutrition.JPEN J Parenter Enteral Nutr. 2017 Nov;41(1_suppl):3S-13S. doi: 10.1177/0148607117742595. Epub 2017 Nov 21. JPEN J Parenter Enteral Nutr. 2017. PMID: 29161196 Review.
Cited by
-
Intravenous lipid emulsions designed to meet preterm infant requirements increase plasma and tissue levels of docosahexaenoic acid and arachidonic acid in mice.Clin Nutr. 2024 Oct;43(10):2273-2285. doi: 10.1016/j.clnu.2024.08.019. Epub 2024 Aug 23. Clin Nutr. 2024. PMID: 39213823
-
Production of Camellia oleifera Abel Seed Oil for Injection: Extraction, Analysis, Deacidification, Decolorization, and Deodorization.Foods. 2024 May 7;13(10):1430. doi: 10.3390/foods13101430. Foods. 2024. PMID: 38790730 Free PMC article.
-
Intestinal failure-associated liver disease model: a reduced phytosterol intravenous lipid emulsion prevents liver injury.Pediatr Res. 2025 Jun;97(7):2454-2461. doi: 10.1038/s41390-024-03753-9. Epub 2024 Nov 27. Pediatr Res. 2025. PMID: 39592772
-
Association between multioil intravenous lipid emulsion and cholestasis in infants with gastrointestinal disorders: A retrospective cohort study.JPEN J Parenter Enteral Nutr. 2025 Aug;49(6):707-716. doi: 10.1002/jpen.2776. Epub 2025 Jun 3. JPEN J Parenter Enteral Nutr. 2025. PMID: 40462537 Free PMC article.
-
Depletion and enrichment of phytosterols in soybean oil lipid emulsions directly associate with serum markers of cholestasis in preterm parenteral nutrition-fed pigs.JPEN J Parenter Enteral Nutr. 2022 Jan;46(1):160-171. doi: 10.1002/jpen.2088. Epub 2021 Apr 30. JPEN J Parenter Enteral Nutr. 2022. PMID: 33581699 Free PMC article.
References
-
- Baker MA, Anez-Bustillos L, Dao DT, Fell GL, Gura KM, Puder M. Parenteral nutrition-associated liver toxicity: prevention, diagnosis, and management. In: Robinson MK, editor Sci Am Medicine. Hamilton, ON: Decker Intellectual Properties; Nov, 2017. Available from: http://www.deckerip.com.
-
- Wanten GJA, Calder PC. Immune modulation by parenteral lipid emulsions. Am J Clin Nutr. 2007;85:1171–84. - PubMed
-
- Li S, Nussbaum MS, Teague D, Gapen CL, Dayal R, Fischer JE. Increasing dextrose concentrations in total parenteral nutrition (TPN) causes alterations in hepatic morphology and plasma levels of insulin and glucagon in rats. J Surg Res. 1988;44:639–48. - PubMed
-
- Ling P-R, Malkan A, Le HD, Puder M, Bistrian BR. Arachidonic acid and docosahexaenoic acid supplemented to an essential fatty acid-deficient diet alters the response to endotoxin in rats. Metabolism. 2012;61:395–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

