Survival Trends in Infants Undergoing Allogeneic Hematopoietic Cell Transplant
- PMID: 30882883
- PMCID: PMC6503511
- DOI: 10.1001/jamapediatrics.2019.0081
Survival Trends in Infants Undergoing Allogeneic Hematopoietic Cell Transplant
Abstract
Importance: Studies demonstrating improved survival after allogeneic hematopoietic cell transplant generally exclude infants.
Objective: To analyze overall survival trends and other outcomes among infants who undergo allogeneic hematopoietic cell transplant.
Design, setting, and participants: In this cohort study, we used time-trend analysis to evaluate 3 periods: 2000 through 2004, 2005 through 2009, and 2010 through 2014. The study was conducted in a multicenter setting through the Center for International Blood and Marrow Transplant Research, which is made up of a voluntary working group of more than 450 transplant centers worldwide. Two groups of infants aged 1 year or younger in 2 cohorts were included: those with malignant conditions, such as leukemia, and those with nonmalignant disorders, including immunodeficiencies. Data analysis was conducted from July 2017 to December 2018.
Exposures: Allogeneic hematopoietic cell transplant.
Main outcomes and measures: Survival trends, disease relapse, and toxicity.
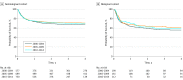
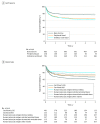
Results: A total of 2498 infants with a median age of 7 months (range, <1-12 months) were included. In the nonmalignant cohort (n = 472), survival rates improved from the first to the second period (hazard ratio, 0.77 [95% CI, 0.63-0.93]; P = .007) but did not change after 2004. Compared with infants with nonmalignant diseases (n = 2026; 3-year overall survival: 2000-2004, 375/577 [65.0%]; 2005-2009, 503/699 [72.0%]; and 2010-2014, 555/750 [74.0%]), those with malignant conditions had poorer survival rates, without improvement over time (3-year overall survival: 2000-2004, 109/199 [54.8%]; 2005-2009, 104/161 [64.6%]; and 2010-2014, 66/112 [58.9%]). From 2000 through 2014, relapse rates increased in infants with malignant conditions (3-year relapse rate: 2000-2004, 19% [95% CI, 14%-25%]; 2005-2009, 23% [95% CI, 17%-30%]; 2010-2014, 36% [95% CI, 27%-46%]; P = .01). Sinusoidal obstruction syndrome was frequent, occurring with a cumulative incidence of 13% (95% CI, 11%-16%) of infants with nonmalignant diseases and 32% (95% CI, 22%-42%) of those with malignant diseases. Generally, recipients of human leukocyte antigen-identical sibling bone marrow grafts had the best outcomes.
Conclusions and relevance: Survival rates have not improved for infants with malignant diseases over the 15-year study period. Infants with nonmalignant diseases had improved survival rates in the earlier but not the later study period. Higher relapses for the malignant cohort and toxicities for all infants remain significant challenges. Strategies to reduce relapse and toxicity and optimize donor and graft selection may improve outcomes in the future.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical