Molecular aspects of bacterial nanocellulose biosynthesis
- PMID: 30883026
- PMCID: PMC6559022
- DOI: 10.1111/1751-7915.13386
Molecular aspects of bacterial nanocellulose biosynthesis
Abstract
Bacterial nanocellulose (BNC) produced by aerobic bacteria is a biopolymer with sophisticated technical properties. Although the potential for economically relevant applications is huge, the cost of BNC still limits its application to a few biomedical devices and the edible product Nata de Coco, made available by traditional fermentation methods in Asian countries. Thus, a wider economic relevance of BNC is still dependent on breakthrough developments on the production technology. On the other hand, the development of modified strains able to overproduce BNC with new properties - e.g. porosity, density of fibres crosslinking, mechanical properties, etc. - will certainly allow to overcome investment and cost production issues and enlarge the scope of BNC applications. This review discusses current knowledge about the molecular basis of BNC biosynthesis, its regulations and, finally, presents a perspective on the genetic modification of BNC producers made possible by the new tools available for genetic engineering.
© 2019 The Authors. Microbial Biotechnology published by John Wiley & Sons Ltd and Society for Applied Microbiology.
Conflict of interest statement
None declared.
Figures


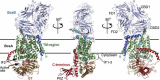

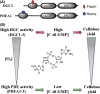

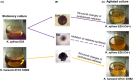
References
-
- Augimeri, R.V. and Strap, J.L. (2015) The phytohormone ethylene enhances cellulose production, regulates CRP/FNRKxtranscription and causes differential gene expression within the bacterial cellulose synthesis operon of Komagataeibacter (Gluconacetobacter) xylinus ATCC 53582. Front Microbiol 6: 633–19. - PMC - PubMed
-
- Ausmees, N. , Mayer, R. , Weinhouse, H. , Volman, G. , Amikam, D. , Benziman, M. and Lindberg, M. (2001) Genetic data indicate that proteins containing the GGDEF domain possess diguanylate cyclase activity. FEMS Microbiol Lett 204: 163–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

